1 ...6 7 8 10 11 12 ...17 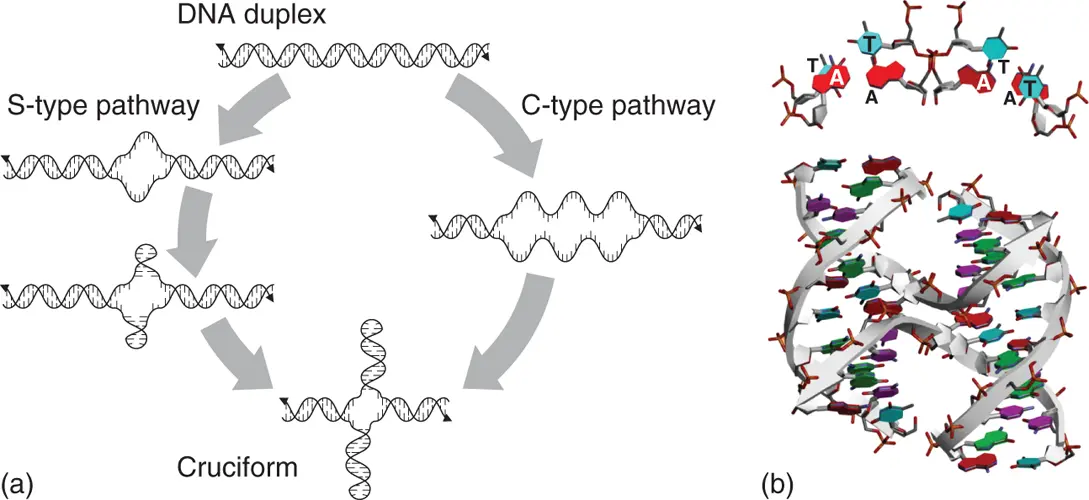
Figure 2.6 Structure of four-way junction to form cruciform and Holliday junction. (a) S-type and C-type pathways to form cruciform structure from long duplex. (b) Structure of Holliday junction formed by DNA strand with 5′-TCGGTACCGA-3′ sequence (PDB ID: 1M6G). Top view of four TA consecutive nucleobases at the region of interstrand exchange are shown above the structure.
2.5 Multi-stranded DNA Helices
Base paring through hydrogen bonds and stacking interactions between the assembled nucleobases are the main factors that maintain the organized structure of nucleic acids. Multi-stranded structures are formed when nucleobases present in different strands more than three interact with each other to form the assembled structure and stably stack over. Figure 2.7shows canonical duplex and other typical multi-stranded helices of nucleic acids, in which structures are resisted in protein data bank, including those consisting of unnatural nucleobases.
Among the multi-stranded nucleic acid structures, triplexes and tetraplexes composed of three and four strands, respectively, have been confirmed in not only aqueous solutions but also intracellular conditions and have attracted attention as non-canonical structures that contribute to gene regulations. Various studies have been conducted on the molecular mechanisms of their influence on the gene expressions from viewpoints of their thermodynamic stabilities and conformational dynamics. The structural characteristics and factors that contribute to the thermodynamic stabilities of triplex and representative tetraplexes, G-quadruplex and i-motif, are described in Chapter 3. The effects of these multi-stranded structures on gene expressions are explained in Chapters 5– 8, focusing on DNA replication including telomeric regions, RNA transcription, and protein translation.
RNA is transcribed from DNA as a single strand, whereas the natural DNA basically exists as a set of complementary strands. The information transformed into functional proteins as a sequence of amino acids is encoded on the primary sequence of RNA. On the other hand, information to modulate the gene expressions exists in their higher-order structures, which are dependent on the secondary and tertiary interactions.
2.6.1 Basic Structure Distinctions of RNA
As described in Chapter 1, by comparing natural RNA to DNA, there are two difference in their chemical structures. One is the ribose sugar, which bears a 2′-hydroxyl group, and the other is uracil nucleobase, which lacks the 5-methyl group of thymidine in DNA. Regarding structure formation, 2′-hydroxyl group of ribose is the most important factor that confers unique conformational features compared to the DNA. The basic feature that RNA forms an A-type duplex is based on the fact that RNA ribose adopts C3′-endo-type conformation in its sugar pucker due to the presence of 2′-hydroxyl group. The constitutive characteristic that RNA is basically single-stranded is also an important element for RNA to form various secondary and tertiary structures.
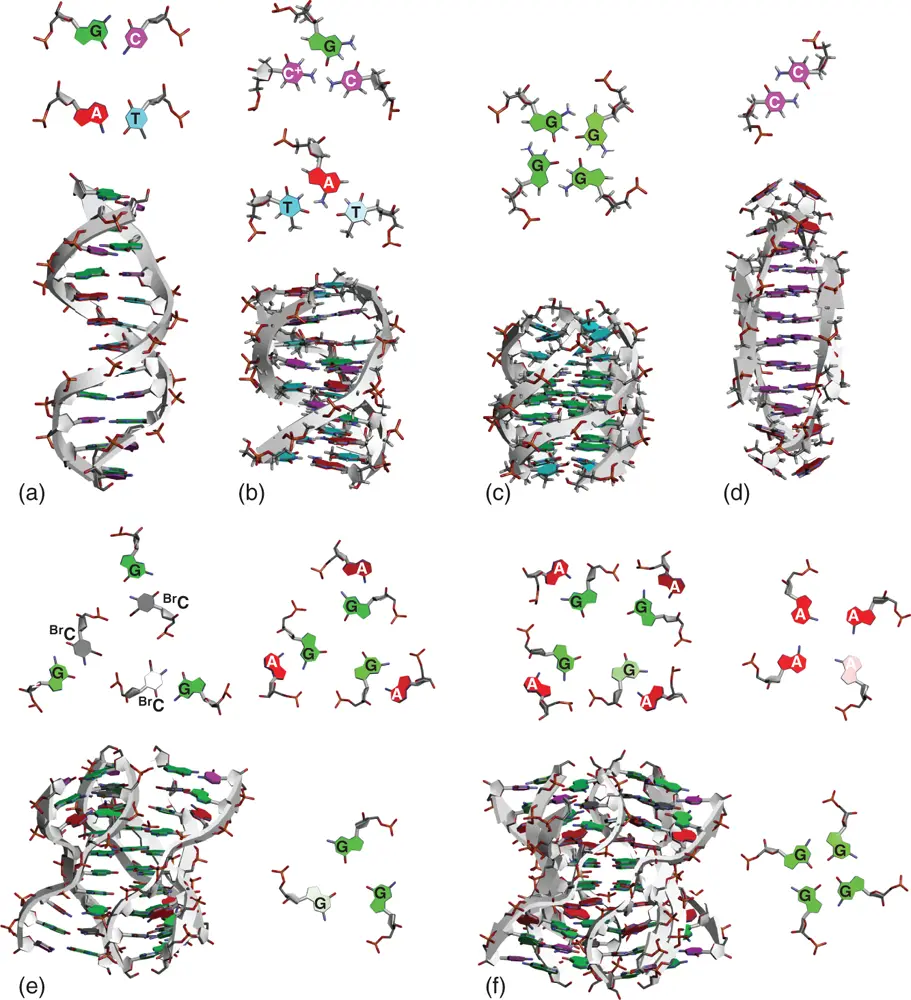
Figure 2.7 Typical structures of multi-stranded DNA helices. (a) Canonical duplex (PDB ID: 1BNA). (b) Triplex (PDB ID: 1D3X). (c) G-quadruplex (PDB ID: 139D). (d) i-motif (PDB ID: 1YBL). (e) DNA hexaplex (PDB ID: 2FZA). (f) DNA octaplex (PDB ID: 1V3P). Top views of nucleobase interactions that are located on coplanar region and important for the formation of multi-stranded helix are shown along with the structure. BrC indicates cytosine modified by bromine.
2.6.2 Elements in RNA Secondary Structures
Single-stranded RNAs basically form their structure based on Watson–Crick-type base pairing within the same strand. At that time, bases not involved in the base pairs remain as single-stranded loop. Depending on how the single-stranded loop region is formed, RNA forms various secondary structures.
Hairpin loop, which is also designated as stem loop, is formed when sequence of RNA has complementary regions at 5′ and 3′ regions. The complementary regions form duplex, and the region in between remains as single-stranded loop. It is an essential secondary structure element not only for interaction of proteins but also for further formation of complexed tertiary structures of RNA. RNA transcripts from the DNA regions, which potentially form cruciform, likely form the hairpin structure. Stability of RNA hairpin depends on the length of loop size. Stability decreases with increasing loop length, and four or five nucleotides in the loop form the most stable hairpin [14]. Closing base pair, which is a base pair located at the boundary between the stem and loop region, also affects the stability. G·C and C·G base pairs are preferred to make stable and robust hairpin structures [15]. Some specific loop sequence, which is typified by UNCG, in which N is any nucleobase, can drastically stabilize the loop region [16]. For example, 5′-UUCG-3′ loop with C·G closing base pair forms one of the most stable hairpin loops, which can be often seen in natural RNAs [17]. NMR structure of 5′-GGACUUCGGUCC-3′ demonstrated that the hairpin loop structure is stabilized by unusual base pairing between uracil and guanine in the loop, in which glycosidic bond angle of guanine is in syn conformation. The structure is also stabilized by a cytosine-phosphate contact and extensive base stacking on the crossing base pair ( Figure 2.8) [18].
Bulge loop is a region in which unpaired nucleotides present on one strand of a continuous duplex. In structures of natural RNAs, the most common bulge loop contains one nucleotide. When single nucleotide bulge loop forms internal stack within the duplex, the helicity of the duplex is distorted, resulting in a kink of the helix axis ( Figure 2.9). When the bulged nucleotide is looped out in solvent, the overall duplex geometries can remain close to the canonical A-form ( Figure 2.9). The bulged nucleobases create unique recognition sites for proteins both directly, by acting as a molecular handle, and indirectly, by distorting the RNA backbone and allowing the proteins access to base pairs in a groove [19]. The kink of the helix caused by the bulge loop also contributes to the shaping of the overall RNA structure. Thermodynamic parameters for single nucleotide bulges indicate that the bulges destabilize the helix. The destabilization effect little depends on the identity of the bulged nucleobase [20]. On the other hand, destabilization does depend on the adjacent base pairs [20b]. As it can be expected, bulge loop with longer nucleotides more destabilizes the RNA structure [21].
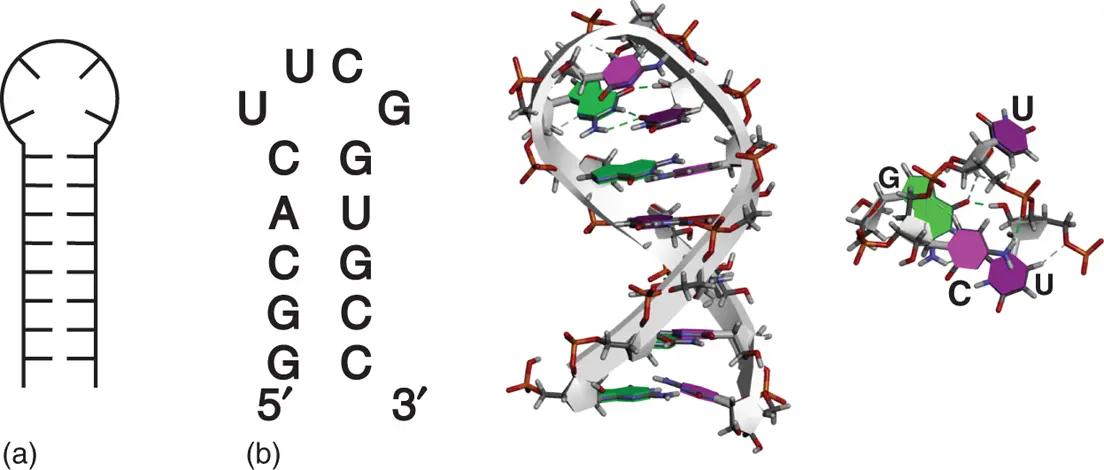
Figure 2.8 Hairpin structure. (a) General secondary structure of hairpin. (b) Sequence and tertiary structure of typical RNA hairpin with stable UUCG tetraloop sequence (PDB ID: 2KOC). Top view of UUCG tetraloop sequence is shown on right side of the structure. Hydrogen bonds formed within the UUCG tetraloop are shown as dashed lines.
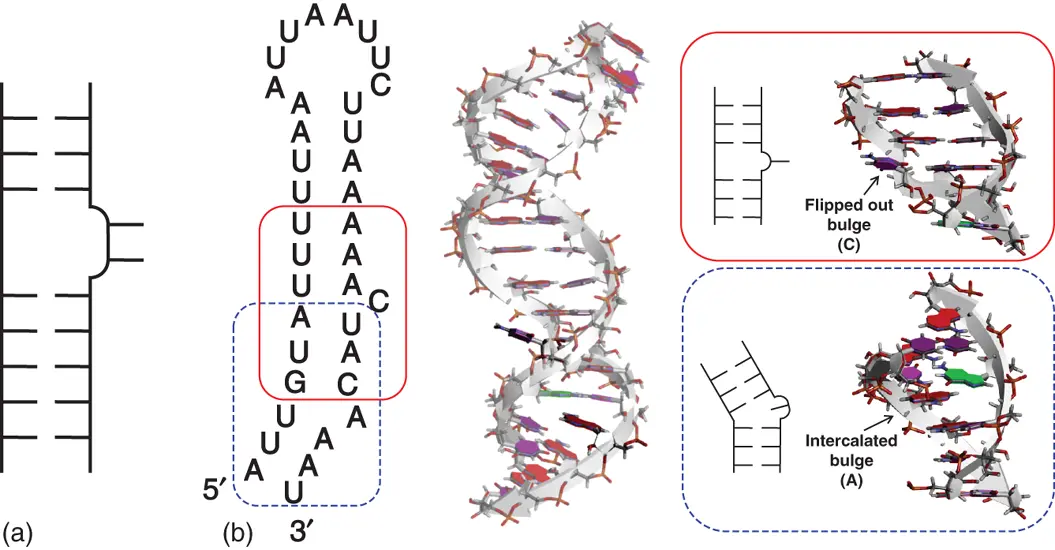
Figure 2.9 Bulge structure. (a) General secondary structure of bulge. (b) Sequence and tertiary structure of RNA hairpin containing single nucleotide bulges, which is derived from an RNA element responsible for dynein-mediated localization of Drosophila mRNA (PDB ID: 2KE6). Bulged nucleobases are emphasized dark. Secondary structure and side view of the region forming single nucleotide bulges are shown in right boxes surrounded by solid and dashed lines.
Читать дальше
















