b)Values are increments of the stabilities (Δ 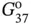 in kcal mol −1) compared with self complementary duplex consisting of CGXX′CG sequence, where X and X′ are nucleobases in the left column.
in kcal mol −1) compared with self complementary duplex consisting of CGXX′CG sequence, where X and X′ are nucleobases in the left column.
c)The sequence has either an unusual conformation or mixture of conformations.
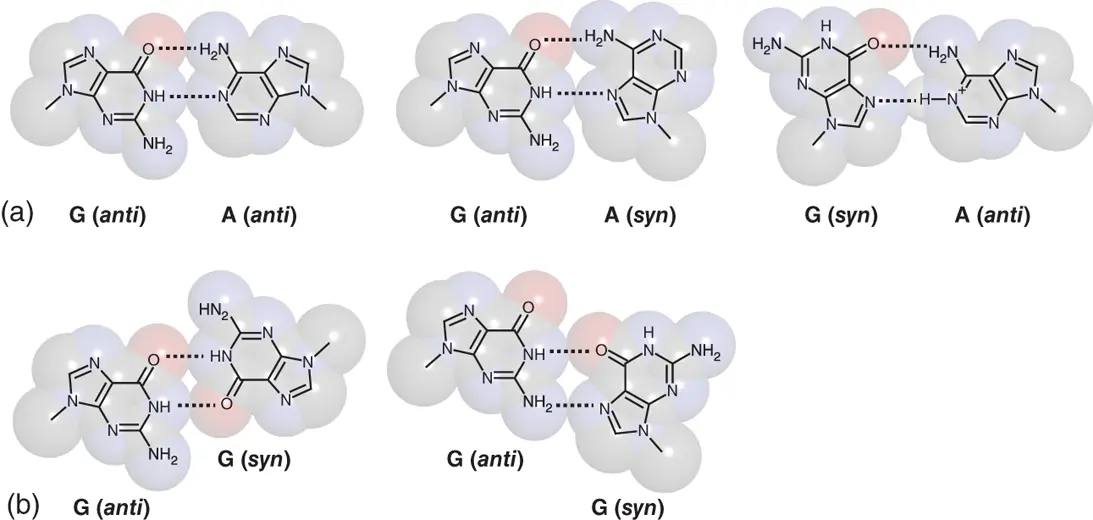
Figure 2.3 Mismatched G-A and G-G base pairs observed in nucleic acid structures. (a) G-A mismatched base pairs with various compositions in their glycosidic bond angles. (b) Symmetric (left) and asymmetric (right) G-G mismatched base pairs.
2.2.4 Pyrimidine–Pyrimidine Mismatches
Pyrimidine–pyrimidine mismatches can be categorized in unstable mismatches ( Tables 2.1and 2.2). When pyrimidine nucleotides base pair through hydrogen bonds, C1 atom of their sugar needs to be in close position. This distorts the duplex backbone and destabilize the structure. However, several hydrogen bonding patterns within the pyrimidine–pyrimidine mismatches have been observed. T·T mismatch adopts by interconverting two base pairing patterns, both of which form two hydrogen bonds with wobble-like configuration ( Figure 2.4). C·T mismatch also forms two hydrogen bonds without wobble orientation. When N3 atom of the cytosine is protonated, C·T mismatch forms two hydrogen bonds with wobble-like configuration similar to the T·T mismatch. Protonation of cytosine also enables formation of two hydrogen bonds in C·C mismatch ( Figure 2.4). If two cytosines, one of which is protonated, are placed on symmetric orientation, C·C mismatch can form three hydrogen bonds. This orientation is not adopted in duplex but observed in tetraplex structure, which is known as i-motif as described in Chapter 3.
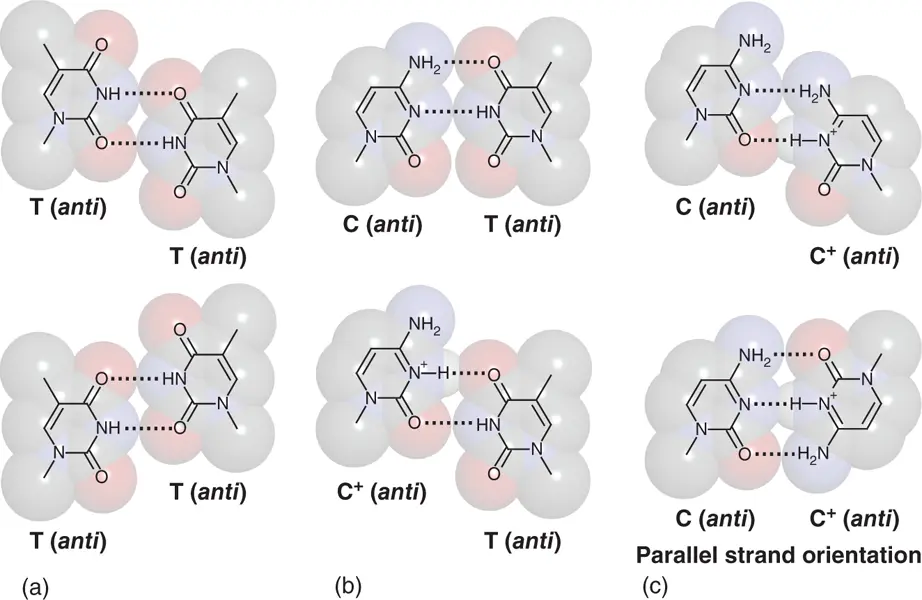
Figure 2.4 Mismatched T-T, C-T, and C-C base pairs observed in nucleic acid structures. (a) Interconvertible T-T mismatched base pairs with wobble-like orientation. (b) C-T mismatched base pairs at neutral form (upper) and a form, in which cytosine nucleobase is protonated (lower). (c) C-C +mismatched base pairs at wobble-like orientation (upper) and symmetric orientation (lower).
2.3 Non-canonical Backbone Shapes in DNA Duplex
As described in Chapter 1, natural DNAs form antiparallel B-type duplex, which is often designated as B-DNA, while natural RNA forms A-type duplex. It is known that DNA duplexes under conditions of low humidity and in solution in which water activity is reduced by the addition of cosolvent such as alcohols form A-type duplex (A-DNA) ( Figure 2.5). X-ray diffraction analysis of oligo-DNA duplexes has demonstrated that many of the duplexes form the A-type ones, especially when their sequences have high G/C contents. However, it has been pointed out that the crystallization conditions of high alcohols commonly used in oligonucleotide crystallography may be factors that provide the A-DNA duplexes. This behavior causes a query whether the A-DNA is the functional structure in biological systems. On the other hand, structure transitions from B-DNA to A-DNA duplex have been demonstrated upon chemical and biological stimuli such as reduction of dielectric constant as one aspect of intracellular crowding condition, addition of polycationic molecule, and binding of proteins related to gene expressions [9]. The B-A transition is actually occurring in the cell nucleus, suggesting that A-DNA duplex would contribute to gene regulation in response to the molecular environment.
Figure 2.5 Structures of A-type (PDB ID: 3V9D) (a) and Z-type (PDB ID: 4OCB) (b) DNA duplexes formed in GC-rich sequence. Top views of consecutive G-C base pairs are shown above the entire structure.
DNA also form Z-type duplex (Z-DNA) ( Figure 2.5). Z-DNA with the two strands forming left-handed helix shows a radically different structure from A- and B-DNAs. Phosphate backbone of Z-DNA forms a distinct zigzag pattern, which is an origin of its name, whereas that of B-DNA and A-DNA is uniformly wound to form the helix. Formation of Z-DNA depends on the oligonucleotide sequence that needs alternating purine–pyrimidine sequence such as d(GCGCGC). Guanine nucleotides in Z-DNA show C3′-end conformation in its sugar puckering and syn orientation in its glyosidic bond angle. These features place the guanine nucleobase back over the sugar ring and make the zigzag pattern in the alternating sequence. Z-DNA has a structure feature that the distance between phosphate groups at interstrand is shorter than B-DNA and A-DNA that results in stronger electrostatic repulsion. Therefore, Z-DNA needs a solution having a high salt concentration or low dielectric constant to be stabilized. It is also known that the Z-DNA is stabilized under the condition with a negative twist when the sequence is incorporated in plasmid DNA and applied in a supercoil state. The biological aspects of Z-DNA have gained more attention after the Z-DNA recognition domain has been discovered in proteins involved in gene regulation. Substantial number of Z-DNA-binding proteins has been identified in various organisms including eukaryotes, prokaryotes, and viruses [10].
2.4 Branched DNA with Junction
Single-stranded DNAs, which have a palindromic sequence in 5′ and 3′ regions in close position, form stable hairpin structure. Even though the sequence is in the duplex such as genomic DNA, addition of negative supercoiling of DNA can loosen the twist of the helix and induce the formation of such intrastranded structures. Cruciform is a structure forming two intrastrand hairpins at opposite positions within the duplex ( Figure 2.6). The region producing the branched DNA structure is called junction. Similar branched DNA structure with the four-way junction is formed during homologous gene recombination that is called Holliday junction ( Figure 2.6). X-ray and NMR studies of the four-way junction showed that the four arms are aligned in pairs to give an oblique “X” structure with continuity of base stacking and helical axes across the junctions. The characteristic of the structure, in which the helices formed by the branched chain coaxially stack each other, is also frequently observed in RNAs having the junction structure. This suggests importance of stacking interaction between the nucleobases that stabilizes the helix even the helices are separated by the junction. Numerous proteins have been shown to interact with the cruciform by recognizing features of the structure such as DNA crossovers, four-way junctions, and curved or bent DNA. Many of them are involved in chromatin organization, transcription, replication, DNA repair, and other processes [11].
Two pathways, S-type and C-type, have been proposed when the cruciform is formed in DNA ( Figure 2.6) [12]. S-type pathway is triggered by small opening, which allows a few bases to make intrastrand base pairs as a nuclear for the branched structure. Formation of the short base pairs is followed by migration and extension of the intrastrand base pairing, which is further facilitated by negative supercoiling. C-type pathway occurs when considerably long region of duplex is melted due to franking AT-rich sequences that allows one-step formation of the mature cruciform. C-type pathway does not probably occur at physiological ion concentrations, but possibly take place in DNA regions with propensities to undergo substantial denaturation such as replication origins [13].
Читать дальше

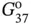 in kcal mol −1) compared with self complementary duplex consisting of CGXX′CG sequence, where X and X′ are nucleobases in the left column.
in kcal mol −1) compared with self complementary duplex consisting of CGXX′CG sequence, where X and X′ are nucleobases in the left column.













