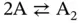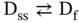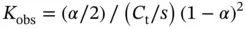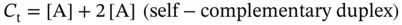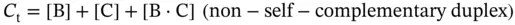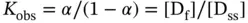
Figure 3.1 Potential hydrogen bonding sites in bases of the nucleosides. Hydrogen bonding donor sites are labeled with red arrows, and hydrogen bonding accepter sites are labeled with blue arrows.
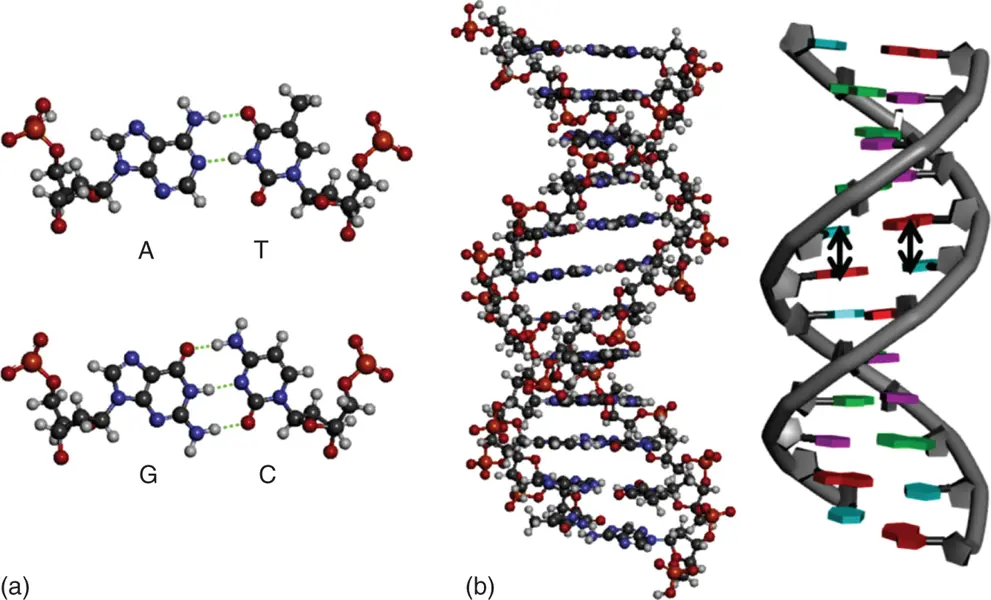
Figure 3.2 (a) Hydrogen bond formation in base pairs in a DNA duplex. The hydrogen bonds are shown in dashed lines. (b) Duplex structures of DNAs are depicted in tube and ball-and-stick models, respectively. Stacking interactions are shown in arrows.
3.2.2 Stacking Interactions
The π–π stacking interactions refer to the interactions between aromatic rings containing π orbitals in bases of nucleic acids. The individual bases make strong stacking interactions with neighboring bases, which are major contributors to duplex stability ( Figure 3.2b). The stacking interactions are much more prevalent in duplexes than in single strands. Base-stacking interactions are London dispersion force interactions and depend on the aromaticity of the bases and their dipole moments. The degree of stabilization afforded by base stacking depends on the DNA sequence. Nearest-neighbor base-stacking interactions are important determinants of duplex stability. The values of free energy change at 37 °C (−Δ 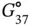 ) due to stacking interactions during duplex formation are 0.4–1.6 kcal mol −1[3].
) due to stacking interactions during duplex formation are 0.4–1.6 kcal mol −1[3].
Moreover, the stacking interactions increase with increasing salt concentration, as high salt concentrations mask the destabilizing charge repulsion between the two negatively charged phosphodiester backbones. The DNA duplex stability increases with increasing salt concentration.
3.2.3 Conformational Entropy
The conformations of nucleotides depend on the torsion angles for rotation around each bonding shown in Figure 3.3. There are seven torsion angles ( α , β , γ , δ , ε , ζ , χ ) per nucleotide that must be specified to characterize the conformation of ordered structures for the nucleic acids. The duplex formation is favorable for enthalpy. However, the loss of conformational entropy is induced by reduction of degrees of freedom for nucleotides.
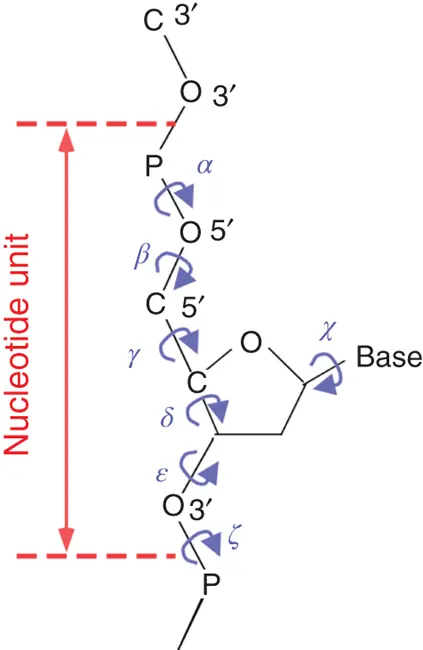
Figure 3.3 Torsion angles in a polyribonucleotide chain.
3.3 Thermodynamic Analysis for the Formation of Duplex
In the case of canonical duplex structures, the base stacking decreases the transition dipole moment of bases, which makes UV absorbance at 260 nm of duplex smaller than that of single-stranded state. Heating of nucleic acids causes the strands to be denatured by disrupting the ordered stacking of the bases and breaking hydrogen bonds. The process can be conveniently monitored by an increase in UV absorbance as the duplex unwinds to single strands owing to hyperchromicity.
The example in Figure 3.4ashows a melting curve (UV absorption as a function of temperature). Slow heating of duplex causes the unwinding of the ordered helical structure into the two single-stranded constituents. The unwinding can be seen as a sigmoidal curve of increasing UV absorption. The midpoint corresponding to the precise melting temperature ( T m) of the duplex is indicated.
Methods to obtain the thermodynamic parameters of enthalpy (Δ H °), entropy (Δ S °), and free energy changes at 25 °C (Δ 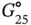 ) for the formation of a nucleic acid structure are described below; DNA duplex formation is taken as an example. Data are typically analyzed with a two-state model, which assumes that each strand is either completely paired or unpaired. The equilibrium for the duplex formation is represented as either a self-complementary or non-self-complementary association as follows [4]:
) for the formation of a nucleic acid structure are described below; DNA duplex formation is taken as an example. Data are typically analyzed with a two-state model, which assumes that each strand is either completely paired or unpaired. The equilibrium for the duplex formation is represented as either a self-complementary or non-self-complementary association as follows [4]:
(3.1) 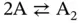
(3.2) 
where A, B, and C indicate the single strands of DNA and A 2and B·C indicate the double-stranded DNA.
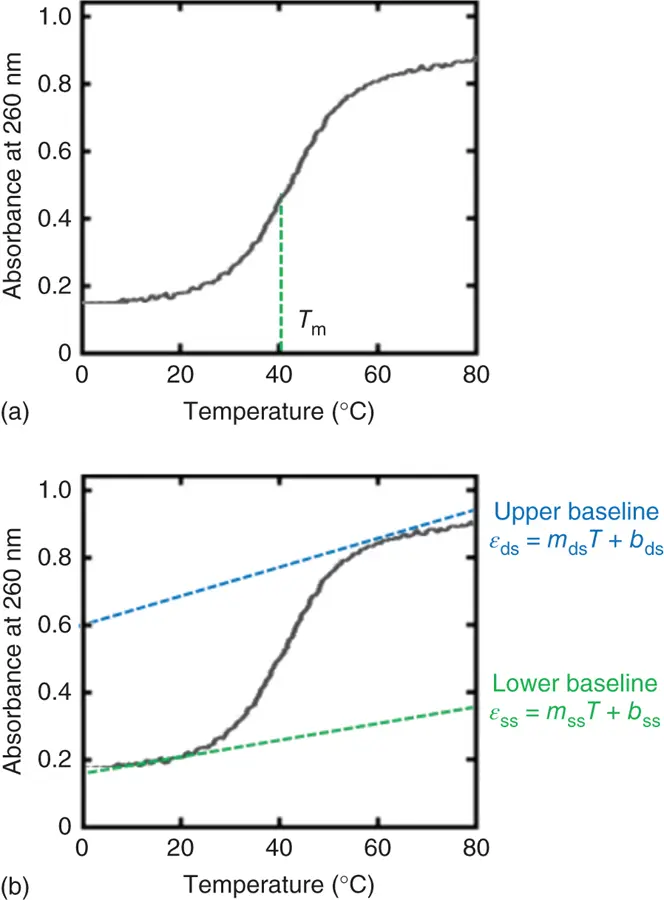
Figure 3.4 UV melting curves of the self-complementary duplex (5′-ATGCGCAT-3′) at 5 μM strand concentration with (a) T mvalue and (b) upper and lower baselines. Upper and lower baselines can be represented as ε ds= m ds T + b dsand ε ss= m ss T + b ss, respectively, where ε dsand ε ssindicate the absorbance for the double-stranded and single-stranded DNA, respectively. The m dsand b dsor m ssand b ssrepresent the slope and intercept of the upper baseline or lower baseline for the UV melting curve, respectively.
The equilibrium for an intramolecular transition, for example, a hairpin, is represented as
(3.3) 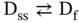
where D ssand D findicate the single-stranded (unfolded) and folded DNA structures, respectively.
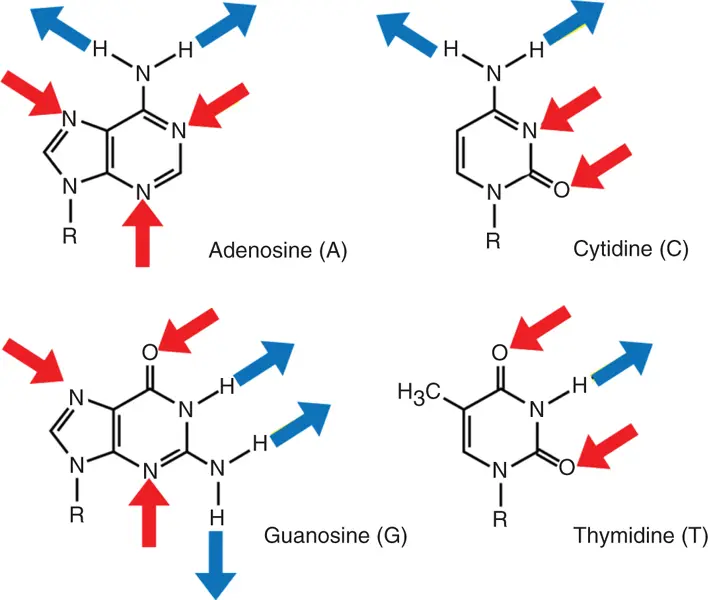
For self-complementary ( Eq. 3.1) or non-self-complementary equilibria ( Eq. 3.2) with equal concentrations of B and C, the observed equilibrium constant K obsis given by
(3.4) 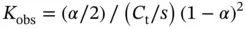
where C tis the total strand concentration, s has a value of 1 for self-complementary duplexes and 4 for non-self-complementary duplexes, and α is the fraction of strands in a duplex:
(3.5) 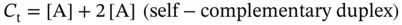
(3.6) 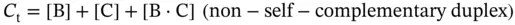
For a unimolecular transition,
(3.7) 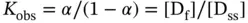
where
(3.8) 
Figure 3.4shows a UV melting curve for the self-complementary duplex (5′-ATGCGCAT-3′). At low temperatures, the strands are in duplex form and the absorbance is low. As the temperature is increased, the duplex dissociates into single strands. The UV absorbance of the duplex is increased by dissociation of the duplex, and the increment of absorbance is referred to as hyperchromicity. (The opposite, a decrement of absorbance, is called hypochromicity.) For self-complementary or non-self-complementary duplexes with equal concentrations of each strand, the melting temperature, T m(in degrees Kelvin), is the point at which the concentrations of strands in duplex and in single strands are equal ( Figure 3.4a). The steepness of the transition indicates the cooperativity of the transition. The width and maximum of the first derivative of the melting curve can also indicate the cooperativity and melting temperature, although the peak of the derivative curve only occurs at the T monly when the transition is unimolecular [5]. T mis most accurately measured by fitting the lower and upper baselines. The melting temperature is measured at several concentrations over a 100-fold range and then plotted versus the concentration in a van't Hoff plot. The van't Hoff equation relates the T m(in degrees Kelvin), C t, Δ H °, and Δ S °:
Читать дальше



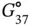 ) due to stacking interactions during duplex formation are 0.4–1.6 kcal mol −1[3].
) due to stacking interactions during duplex formation are 0.4–1.6 kcal mol −1[3].
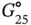 ) for the formation of a nucleic acid structure are described below; DNA duplex formation is taken as an example. Data are typically analyzed with a two-state model, which assumes that each strand is either completely paired or unpaired. The equilibrium for the duplex formation is represented as either a self-complementary or non-self-complementary association as follows [4]:
) for the formation of a nucleic acid structure are described below; DNA duplex formation is taken as an example. Data are typically analyzed with a two-state model, which assumes that each strand is either completely paired or unpaired. The equilibrium for the duplex formation is represented as either a self-complementary or non-self-complementary association as follows [4]: