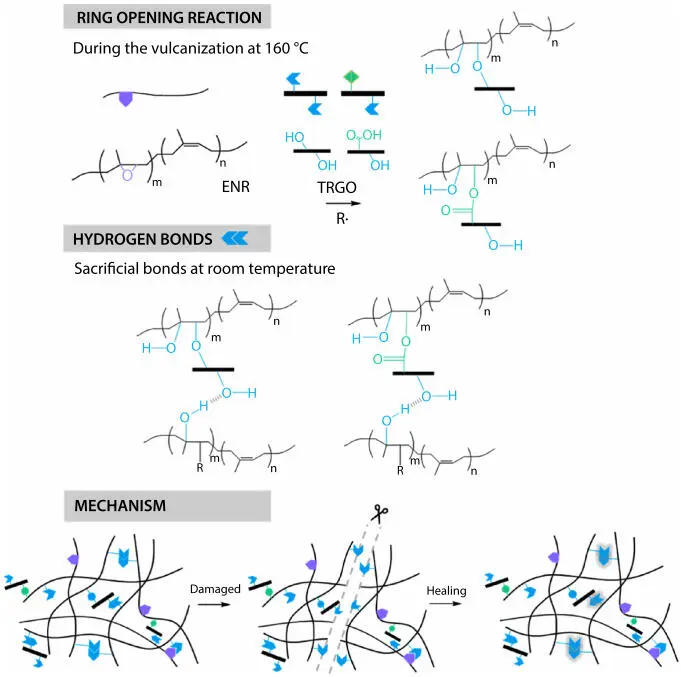
Figure 3.11Proposed scheme of self-healing mechanism in epoxidized natural rubber and thermally reduced grapheme oxide composites (ENR/TRGO) (Reprinted with permission from Utrera-Barrios et al . [46]. Copyright 2020 American Chemical Society).
3.3.2 Styrene Butadiene Rubber (SBR)
Styrene butadiene rubber is the most used system among the synthetic rubbers. Due to the huge amount of scrap from tires, some researchers focused its interest on the self-healing behavior, which will allow to increases the time of use and the development of high performance smart tires.
Hernandez Santana et al. analyzed the self-healing behavior in SBR compounds containing ground tire rubber (GTR) particles and the coupling agent bis[3-(trietoxysilyl)propyl] tetrasulfide (TESP) [50]. Due to healing process requires chain mobility, authors prepared samples varying the accelerant/sulfur ratio (A/S) in order to evaluate the influence of the density and type of crosslinks: mono, di and polysulfides [51]. The sulfur amount was fixed in 0.7 phr.
The healing efficiency was evaluated through tensile tests: once the test was performed, the two pieces were repositioned together at 70 °C in a press at 10 bar for 7 h. Afterwards, the sample was tested again and the obtained results are shown in Figure 3.12. It was observed that systems with the lowest tensile strength exhibit the higher healing efficiency, corresponding to the ratios A/S = 0.2 and 1. The healing efficiency is attributed to the chain entanglement between the dangling chains on each piece, followed by thermal scission of the di and poly-sulfide bonds, which increases the chain mobility in the broken area. Also was confirmed that the healing efficiency is strongly reduced if the chain mobilization decreases due to a denser crosslinking network or by the use of reinforcement particles.
Araujo-Morera et al. [52] incorporated different amounts of GTR (10, 20 and 30 phr) into an SBR compound. The samples were submitted to 20 stretch cycles up 70 % strain in each cycle. After that, each sample was stored 12 h at room temperature and 12 h at 70 ºC. Through dynamic mechanical analysis (DMA) it was stated that the elastic modulus (E’) diminishes in all the samples once the damage process was applied. That behavior is attributed to the Mullins effect, which is a result of different contributions like: chain scission, breaking of crosslinks, chains disentanglement, filler deagglomeration and softening of the chains that were near the filler surface (bound rubber) [53]. Once the healing protocol was applied, an efficiency of 110 % was obtained in the unfilled sample, which is attributed principally to the entanglement of the dangling chains and to the re-crosslinking of the damaged poly and disulfide crosslinks. Same authors used dielectric spectroscopy to analyze the type of interaction within the rubber composites. Figure 3.13 shows dielectric spectrum of different samples at −25 °C. In the case of the unfilled sample, either in the virgin and damage states, a similar spectrum was obtained, which indicate that the relaxation processes of the sample were exactly the same (Figure 3.13a). After the healing protocol, the spectrum presents a slight widening at high frequencies, indicating a higher number of entanglements promoted by the thermal healing treatment. In the case of the compound with 10 phr of GTR, a particular behavior was observed in Figures 3.13b and c, since the virgin sample indicates the presence of a percolated network that is broken with the cycled deformation, which cannot be recovered after the healing treatment.
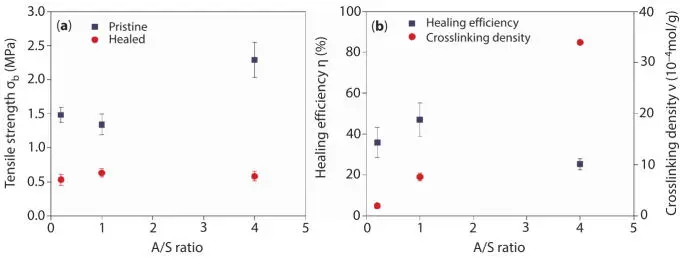
Figure 3.12SBR vulcanized with different values of the ratio accelerant/sulfur (A/S): (a) Tensile strength of pristine and healed samples and (b) Healing efficiency and crosslink density as function of A/S (Reprinted with permission from Hernandez Santana et al . [50]).
Kuang et al. [38] crosslinked furfuryl grafted SBR (SBR-FS) with bismaleimide (M 2) via DA reaction at different molar ratios (1/1, 2/1 and 3/1). After the tensile test, the broken samples were pressed and healed at different thermal conditions (70 °C for 30 min, 100 °C for 30 min and 100 °C for 5 h) to induce the healing behavior giving by the reversibility of the DA/ rDA reaction. In addition, author included carbon nanotubes functionalized with furfuryl groups (MWCNT-FA) were included in the compound.
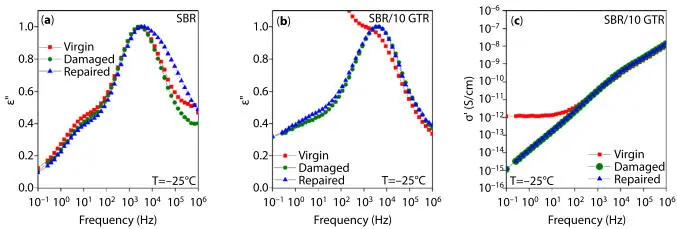
Figure 3.13Dielectric parameters (e” and s”) as function of frequency at −25 °C: (a) unfilled SBR and (b, c) SBR/10GTR compound (Reprinted from Araujo-Morera et al . [52], open access).
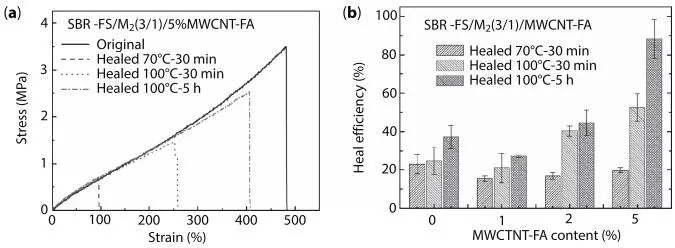
Figure 3.14(a) Stress–strain curves of SBR-FS/M2 = 3/1 with 5% of MWCNT-FA and (b) Healing efficiency as a function of MWCNT-FA; both for the original sample and healed samples at the specified healing conditions (Adapted with permission from Kuang et al. [38]).
Figure 3.14(a) exhibits the stress–strain curves of samples whose molar ratio was 3/1 of furan/maleimide and 5% of MWCNT-FA. It can be observed that the stress–strain curves with the 3 different conditions after the healing process are overlapped. Moreover, the healing efficiency increases with temperature and time during which the temperature is applied. In Figure 3.14(b) is observed that a small addition of 1 phr MWCNT reduces the healing efficiency.
3.3.3 Polybutadiene Rubber
The other important synthetic rubber in the tire industry is polybutadiene rubber (BR) due to his high resistance to wear and low rolling resistance.
Xiang et al. analyzed samples of BR compounded with a liquid polysul-fide that provides disulfide bonds and thiol end groups [54]. The curing (vulcanization) and self-healing processes was promoted through UV radiation at room temperature. The authors emphasize that a photo induction method requires less energy than that based on thermal activation. A disulfide metathesis by including diethyl disulfide (DEDS) and dibutyl disulfide (DBDS) was used due to this mechanism is promoted by the UV radiation and the metathesis was characterized at different irradiation intensities and time intervals. The amount of the liquid polysulfide was varied between 50, 100 and 150 g in 100 g of rubber, naming the compounds B1S5, B1S10 and B1S15 respectively. Through gas chromatography–mass spectroscopy it was observed that increasing the UV intensity the time require to reach the metathesis equilibrium diminishes and the optimum value for curing was determined in 20 mW/cm 2.
The self-healing was evaluated through tensile tests: after the failure the samples were put in physical contact and a radiation intensity of 20 mW/ cm 2was applied during 3 h. It was found that the compound B1S10 reaches the highest healability with an efficiency of ~60 %. Then, in this compound were applied different intensities between 5 and 40 mW/cm 2for 2 h, determining that the optimum intensity is 30 mW/cm 2as can be seen in Figure 3.15a. Then with that radiation amplitude the exposure time was varied from 0.5 up to 3 h, founding that the healing efficiency increases with the time, reaching a 97% (Figure 3.15b). Due to the reversibility of the disulfide metathesis the healing mechanism can be applied more than once. As can be seen in Figure 3.15c, efficiency higher than 90% is maintained even after the third cycle of healing.
Читать дальше
















