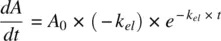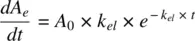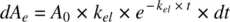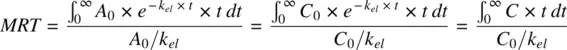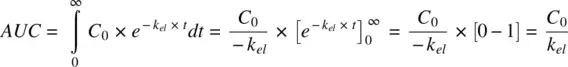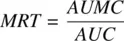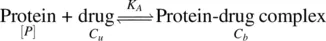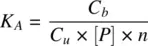Knowing the dose administered and the AUC , clearance can be calculated using Equation 1.10. The volume of distribution, V , of the drug can be determined using Equation 1.11, knowing that clearance is the product of k eland V .
(1.11) 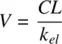
Most small molecule drugs bind reversibly to plasma proteins such as albumin and alpha‐glycoprotein. Drug binding to plasma proteins is of major interest in pharmacokinetics as it impacts both clearance and volume of distribution. Thus far, the term clearance refers to blood clearance. However, measurements of drug concentrations are often done in plasma, as whole blood contains cellular elements (red and white blood cells, platelets etc.) and proteins (albumin, glycoproteins, globulin, lipoproteins etc.). The clearance of a drug determined using the AUC estimated from plasma drug concentration‐time profile is referred to plasma clearance. To convert plasma clearance to blood clearance, the distribution of a drug between blood and plasma should be measured. The ratio of drug concentrations in blood to plasma is known as blood–plasma ratio ( R ).
Mean residence time is a parameter closely related to half‐life and is defined as the average time drug molecules spend in the body before being eliminated. It is expressed as the sum of the residence times of all drug molecules, divided by the total number of molecules. If dA eis the number of drug molecules exiting the body at time interval t , MRT is given by:
(1.12) 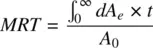
Differentiating Equation 1.5( 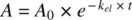 ),
),
(1.13) 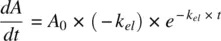
Recognizing that the rate of decline in A = − rate of amount exiting the body, A e:
(1.14) 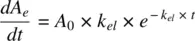
(1.15) 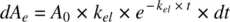
Substituting for dA eusing Equation 1.15in Equation 1.12and dividing both numerator and denominator by k el, we get
(1.16) 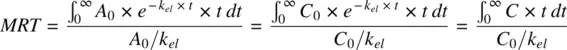
The numerator of equation is the first moment of the concentration–time integral, or the area under the curve formed by time and the product of concentration and time, also called the area under the first moment curve ( AUMC ). The denominator of equation is the same as AUC as shown below:
(1.17) 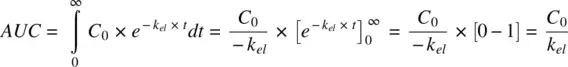
Thus, MRT for an IV bolus is given by the ratio of AUMC and AUC
(1.18) 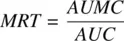
1.2.3 Plasma Protein Binding and Blood–Plasma Ratio
Drugs reversibly bind to plasma proteins depending upon their lipophilicity and ionizability. In general, the greater the lipophilicity of a compound, the greater its extent of plasma protein binding. The binding equilibrium can be represented as:
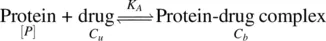
[ P ] is protein concentration; C uand C bare the unbound and bound concentrations of the drug at equilibrium. The equilibrium constant, K A, also called the affinity constant is given by
(1.19) 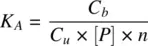
n is the number of binding sites per mole of the binding protein. Since the therapeutic concentrations of most drugs are low relative to the total protein concentration, [ P ] can be assumed to be the total protein concentration [ P ] Total. The fraction unbound in plasma ( f up) can be obtained from Equation 1.20in terms of [ P ] Totalor in terms of the concentrations of α1‐acidic glycoprotein (AGP), [ P ] AGPand albumin [ P ] albumin:
(1.20) 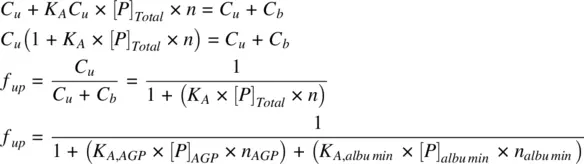
The fraction unbound in plasma ( f up) thus depends on the concentrations of plasma proteins and the affinity of the drug to the plasma proteins. Albumin is the principal protein to which many drugs bind, followed by AGP. Other plasma proteins include lipoproteins and globulins. The concentrations of various plasma proteins are shown in Table 1.1. Albumin is distributed in intravascular (plasma: 43 g/kg organ) and extravascular organs (muscle: 2.3 g/kg, skin: 7.7 g/kg, liver: 1.4 g/kg, gut: 5 g/kg, and other tissues: 3 g/kg). Albumin exists abundantly in the interstitial fluids.
TABLE 1.1. Plasma proteins.
| Plasma proteins |
Binding |
Molecular weight (Da) |
Concentration (μM) |
| Albumin |
Binds mainly to anionic compounds |
67 000 |
500–700 |
| α1‐acidic glycoprotein (AGP) |
Binds mainly to cationic drugs. E.g.: tricyclic antidepressants |
42 000 |
9–23 |
| Lipoproteins |
|
200 000 |
Variable |
| α‐, β‐ and γ‐globulins |
|
53 000 |
0.6–1.4 |
Albumin has six distinct binding sites, two of which specifically bind to long‐chain fatty acids, another selectively binds to bilirubin and two others bind to acidic and lipophilic drugs. One of these two drug binding sites binds drugs like warfarin, and phenylbutazone, while the other binds drugs such as diazepam and ibuprofen. Drugs binding to different binding sites do not compete with one another. When more than 20% of the sites are occupied, concentration dependence of binding begins to get appreciable, ultimately leading to saturation at higher concentrations. Saturation of albumin is rare and restricted to drugs (especially acids) with high therapeutic concentration. However, the binding sites of a few drugs such as tolbutamide and some sulfonamides are saturated even at therapeutic concentrations. AGP concentrations being much lower compared to albumin, saturation of AGP occurs at lower therapeutic concentrations. The concentrations of several plasma proteins can be altered by many factors including stress, surgery, liver dysfunction, and pregnancy. Most commonly, disease states increase AGP concentration while reducing albumin concentration. Higher levels of AGP have been reported in obese patients with nephrosis. Stress, cancer, and arthritis have been associated with lower AGP levels. Neonates have higher AGP levels. AGP is associated with a higher inter‐individual variability compared to albumin. Reduced levels of albumin have been reported in myalgia patients. Drugs that are highly bound to plasma proteins are confined to the vascular space and are not readily available for distribution to other tissues and organs. Many carboxylic acid drugs are not easily displaced from plasma proteins and have a low distribution volume. However, this is not true if the affinity of a drug to tissue proteins is higher than that to plasma proteins. Ultrafiltration and equilibrium dialysis are the two commonly employed methods for the determination of plasma protein binding (Wright et al., 1996). Albumin is the principal drug‐binding protein in tissues followed by ligandin. Measurement of tissue binding is not as straight forward as that in plasma, as the tissue must be disrupted, and it is not readily accessible for sampling. Tissue proteins cannot be easily separated into its constituents and cannot easily be quantified.
Читать дальше

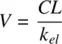
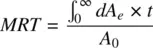
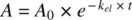 ),
),