Sheila Annie Peters - Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulations
Здесь есть возможность читать онлайн «Sheila Annie Peters - Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulations» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulations
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulations: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulations»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
The first book dedicated to the emerging field of physiologically based pharmacokinetic modeling (PBPK) Physiologically Based Pharmacokinetic (PBPK) Modelling and Simulations: Principles, Methods, and Applications in the Pharma Industry
Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulations: Principles, Methods, and Applications in the Pharmaceutical Industry, Second Edition
Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulations — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulations», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Data from genomics and proteomics are helping scientists to differentiate between healthy and disease states, leading to biomarker discovery (Colburn and Keefe, 2003). Most biomarkers are endogenous macromolecules which are measured in biological fluids such as whole blood plasma, serum, urine, saliva buccal mucosa samples, sweat or tissues, tumor etc. A biomarker must be measurable in preclinical models and patients (translatable). An analytical validation of a biomarker assay is needed to establish its performance characteristics (good precision, resolution, dynamic range, sensitivity) robustness and reproducibility (Colburn and Lee, 2003) ( Figure 1.16). To improve the signal‐to‐noise ratio, biomarker levels measured under treatment should be normalized against baseline data either by subtraction or division. An ideal biomarker is one that permits dense, longitudinal and easy sampling (low volumes of samples collected at frequent timepoints) and non‐labile sample handing. It should ideally change rapidly in a short time so that it can be implemented in Phase I/II. It should be possible to sample, measure, and quantify the biomarker both at baseline and on‐treatment. These requirements are summarized in Figure 1.17a. PD biomarkers may be proximal or distal proteins (preferably in target tissue) in the targeted pathway that shows modulation upon treatment ( Figure 1.17b). They allow ‘Go’/‘No Go’ development decisions based on evidence of achieving target engagement or desired biological activity. They can support dose selection for expansion cohorts as well as for pivotal trials and dose scheduling based on PD half‐life.
TABLE 1.6. Examples of different types of biomarkers.
| TYPE 5 biomarker | Surrogate marker | Clinical endpoint | |
|---|---|---|---|
| Definition | A characteristic that is objectively measured and evaluated as an indicator of normal biologic process or pharmacologic response | A biomarker intended to substitute for a clinical endpoint and expected to predict the effect of a therapy. Selection of surrogate is based on epidemiologic, therapeutic, pathophysiologic or other scientific evidence for predicting clinical endpoint | A characteristic or variable that reflects how a patient feels or functions or how long a patient survives |
| Value | Mainly in early efficacy and safety evaluation in in vitro studies, in vivo animal models, and early clinical trials to establish proof of concept. Need not be directly related to clinical outcome | Biomarkers that are readily observed and easily quantified. Predicts clinical outcome. Clinical relevance of the surrogate is generally well validated | Assess benefit (cure or reduced morbidity) of therapeutic intervention to the patient. Ultimate measure of efficacy, but difficult to quantify. |
| Examples | Blood cholesterol concentrations for assessing risk of heart disease. Receptor occupancy Extent of target modulation | Pupil dilation for narcotics, biochemical tumor markers for anticancer drugs, exercise tolerance tests in chronic stable angina, for myocardial infarction. QT interval as a surrogate for Torsades de Pointes. HbA1c for diabetes. Blood pressure, body weight for obesity; Viral load of HIV, hepatitis C or B virus for assessing level of infection. CD4 cell counts. | Chest pain for a medication aimed at prevention of heart attack Overall survival for cancer indications Recurrence of cancer, stroke Occurrence of infections in HIV |
| Biochemical and clinical biomarkers | |||
| Disease | Biochemical biomarker | Clinical biomarker | |
| Asthma and chronic obstructive pulmonary disease | Leukotrienes, chemokines, and cytokines | Pulmonary function tests, exacerbations | |
| Type 1 or 2 diabetes; Diabetic retinopathy/nephropathy | Glucose, fructosamine, glycosylated albumin glycated hemoglobin (HbA1c), and cytokines | Retinal evaluation, nephropathy measures, and peripheral neuropathy assessments | |
| Hypertension | Angiotensin I, angiotensin II, plasma renin, aldosterone, and ACE activity | Blood pressure and heart rate measures |
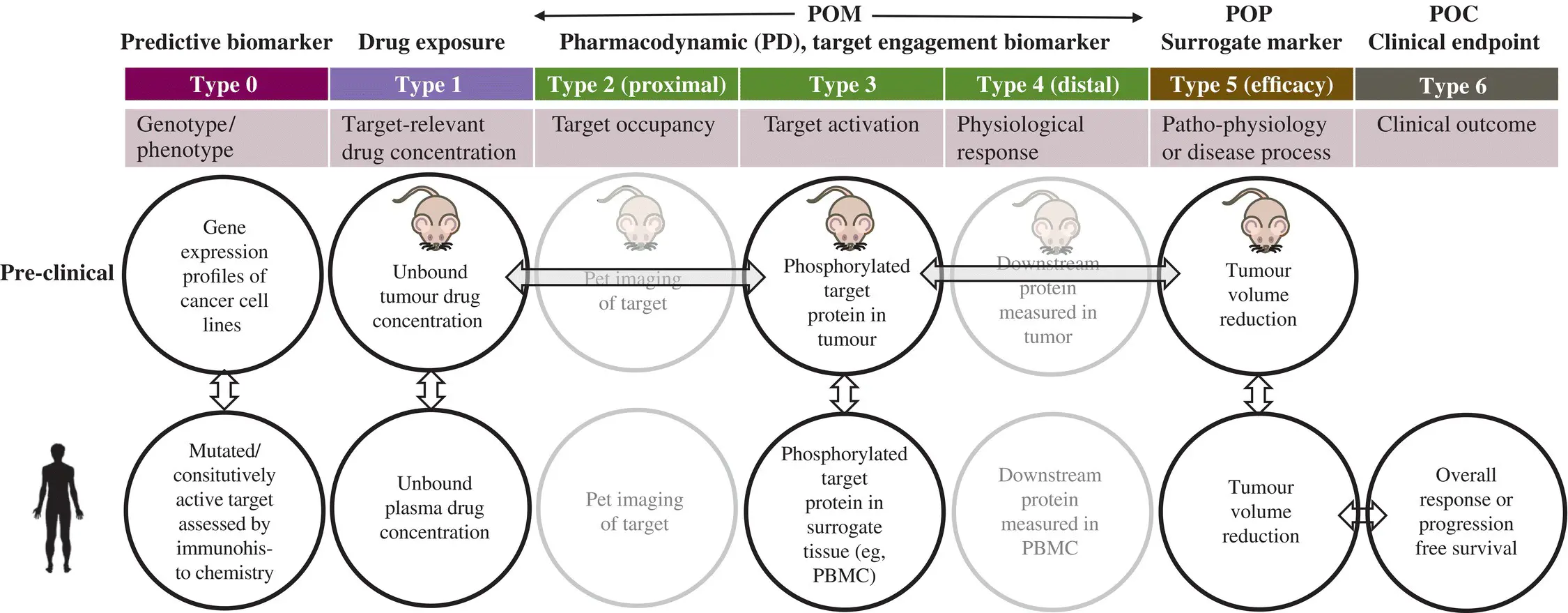
Figure 1.15. Biomarker classification (Types 0–6) and typical preclinical, clinical biomarkers in a drug development project in oncology. Biomarkers enable demonstration of target engagement, modulation of patho‐physiology or disease process and clinical efficacy that is needed for proof of mechanism (POM), proof of principle (POP), and proof of concept (POC) respectively, in preclinical models or clinical phases of drug development. A PK/PD model correlating the unbound drug concentrations at the target (tumor) to the target engagement biomarker is represented by horizontal double‐headed arrow. A translated PK/PD model could be useful to assess target engagement and guide dose escalation studies. A PD‐efficacy correlation in preclinical model identifies the extent of PD modulation needed for efficacy. Assuming that the extent of target engagement needed for efficacy is the same across species, the dose needed for clinical efficacy is derived from a translated PK/PD model. Alternatively, the correlation of tumor growth rate inhibition in xenograft mouse model to overall response in human (represented by vertical double‐headed arrow) may be used along with a PK‐efficacy model to identify human efficacious dose.
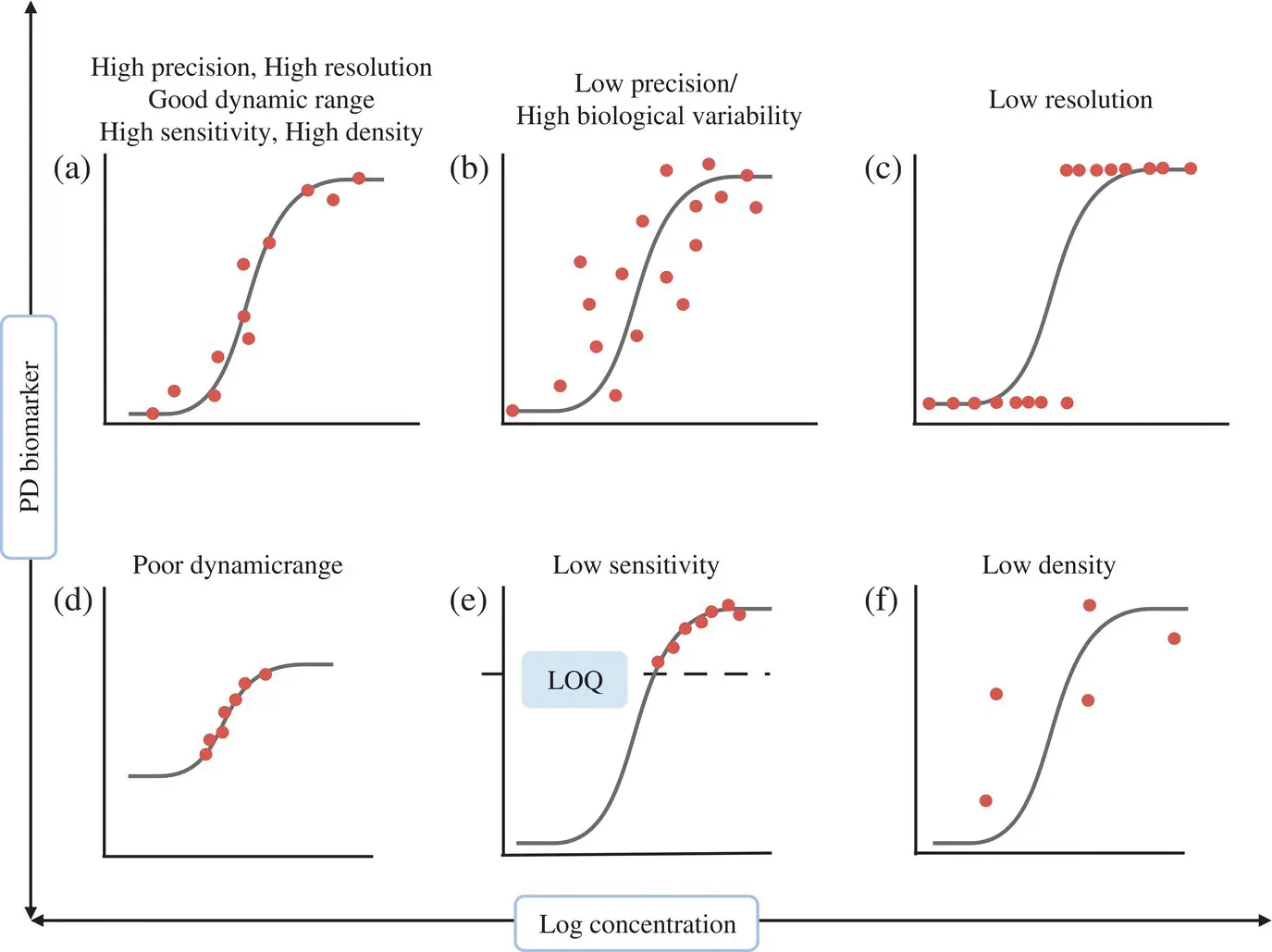
Figure 1.16. Characteristics of an ideal biomarker. (a) A biomarker with ideal characteristics can predict effect from exposure and can guide dose escalation. (b) Poor precision due to assay method, biological variability, or sample handing variability and intersite variability due to lability. More data points are needed to correlate exposure to effect for individual subjects. (c) Categorical rather than continuous effects may be due to assay method or biology. A binary read out is sufficient to demonstrate proof of mechanism but not to explore exposure–effect relationship. (d) Poor dynamic range typical for protein quantification with immunohistochemistry or ELISA unlike mRNA assays. (e) Low biological or low assay sensitivity in the biologically relevant region. LOQ: limit of quantification. (f) Sparse sampling (typical for biopsy‐based markers) (Source: Colburn and Lee, 2003).
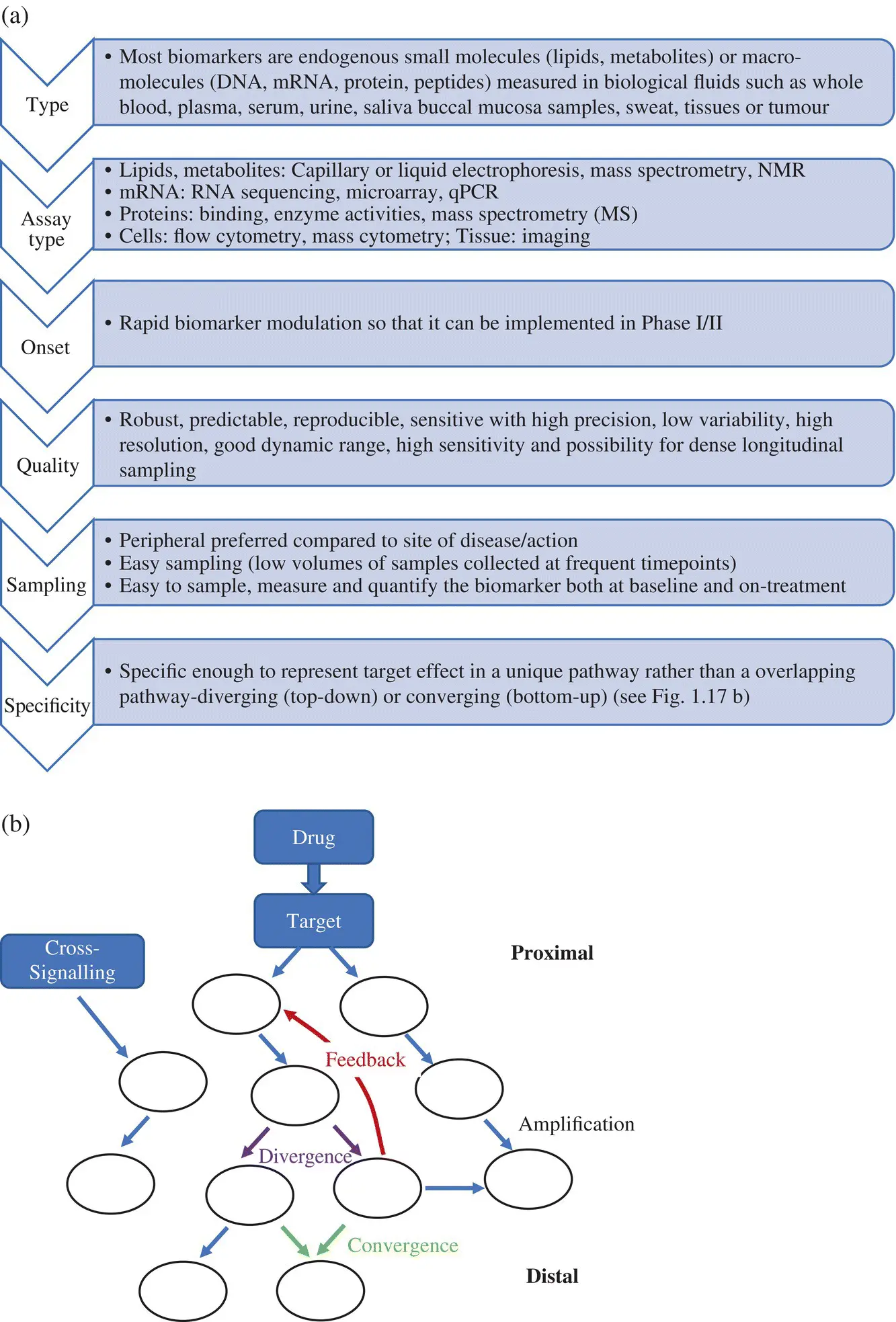
Figure 1.17. Pharmacodynamic biomarkers. (a) Types of biomarkers, assays for these different types and desired features for a biomarker. (b) Proximal biomarkers are those that measure events close to drug‐target binding. Distal biomarkers measure events that are far from the drug‐target binding and are useful for validating mechanism of action. Multiple steps could intervene between target engagement and the measured distal biomarker, each with its unique time course of onset, duration, and offset of response. Some of these may be divergent or convergent pathways.
KEYWORDS
Biomarkeris a characteristic that is readily detectable in plasma or cellular molecules (typically gene mutations or proteins) objectively measured and evaluated as an indicator of normal biological processes pathogenic processes or pharmacologic responses to a therapeutic intervention Desensitization:agonist‐induced loss of receptor responsiveness triggered by a persistent exposure of receptors to an agonist by any mechanism – receptor phosphorylation, uncoupling, antagonistic metabolites, or negative physiological feedback that occurs over a relatively short period, receptor downregulation, or receptor internalization or sequestration Efficacy:A drug’s ability to bind a receptor and elicit a functional response. Enterohepatic recirculation(EHR):refers to the circulation of bile components secreted from the liver, into the small intestine, from where it can be reabsorbed again. EHR contributes to an increased half‐life of a drug. Some phase II conjugates are also secreted into bile and converted back to parent in the distal small intestine and reabsorbed as parent. Glomerular filtration rate(GFR):is the rate at which water is filtered out of the plasma through the glomerular capillary walls into Bowman's capsules, usually measured by the rate of clearance of creatinine. Internalization:is the sequestration of agonist–receptor complexes into endosomal compartments within cells. This is important since removal of receptors from the plasma membrane prevents further stimulation by intercellular signals. Irreversible inhibitoror antagonist (as opposed to reversible):covalently modifies and inactivates the target protein by forming irreversible complex and having bound to the receptor, dissociates from it very slowly or not at all. Example: alkylating agents. Irreversible inhibitors are characterized by low turnover of effect or a long response half‐life and are long acting by virtue of receptor occupancy rather than PK half‐life. Duration of effect is determined only by turnover of target protein. Lead optimization:is a stage of the drug discovery phase, when a preclinical drug candidate is identified from one or more lead series through iterative cycles of compound synthesis, testing, and design. The identified lead should satisfy a predefined criteria with respect to novelty, potency, selectivity, physicochemical characteristics, pharmacokinetics, and toxicity. Polymorphism, polymorphic enzymes:Polymorphism is a discontinuous genetic variation in proteins/enzymes that occurs in at least 1% of a population, leading to different types of individuals in a single species. Predictive biomarker(also called stratification biomarker):is a pretreatment marker (genotype or phenotype that predicts who will/will not benefit from a therapy). This is the basis for a companion diagnostic. Rebound:Occurs when the body tries to return to homeostasis following a sudden discontinuation of a drug. Receptors which are suddenly deprived of their agonists/blockers become hypersensitive to an agent that targets it, causing a further exacerbation of the symptoms/conditions that triggered the use of the drug in the first place. This rebound effect can be minimized by a gradual rather than a sudden discontinuation of the drug. Second messengers:Relay molecules that target other molecules in cytosol and/or nucleus in response to receptors that are activated at the cell surface by hormones, growth factors etc. They can greatly amplify the strength of a signal by causing large changes in the biochemical activities within the cell. Examples of second messengers include cyclic nucleotides like cAMP and cGMP, inositol trisphosphate (IP3), diacylglycerol (DAG), and calcium ions. Sensitization:is defined as an increased effect of drug following repeated doses. Drug‐induced sensitization is the opposite of tolerance. Sensitization can result from accumulation of parent or active metabolites, positive feedback, upregulation of receptors, or enhanced sensitivity of receptors to a ligand. Steric hindrance:is the reduction in metabolic activity of a molecule due to bulky chemical groups close to the site of metabolism in a new chemical entity. Surrogate biomarkeror endpoint:is a biomarker intended to substitute for a clinical endpoint. In a clinical trial, it is a laboratory measurement, or a physical sign used as a substitute for a clinically meaningful endpoint. Target engagement:describes how robustly a drug interacts with the mechanism of action. Tolerance:is loss of receptor‐activated function, following prolonged exposure to a drug, usually due to receptor downregulation. Tolerance is reserved for reduced responsiveness developing over longer period while desensitization over shorter period.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulations»
Представляем Вашему вниманию похожие книги на «Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulations» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulations» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












