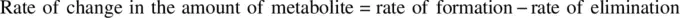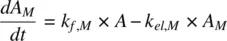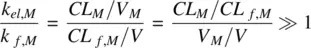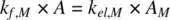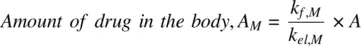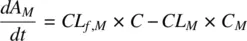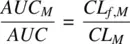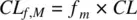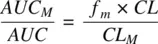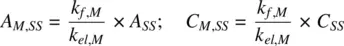
(1.50) 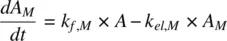
k f,Mand k el,Mare the first‐order rate constants of metabolite formation (strictly, metabolite entry into systemic circulation) and elimination respectively. For simplicity, the metabolic pathway leading to the active metabolite is the only elimination pathway for the parent drug and a complete release of the metabolite into systemic circulation without prior elimination in the organ where it is formed is assumed.
1.2.10.4 Limiting Conditions
Equation 1.50suggests three different possibilities:
1 Metabolite disposition is formation rate‐limited (kel,M > kf,M ) – commonly observed
2 Metabolite disposition is elimination rate‐limited (kf,M > kel,M )
3 Neither of the two steps above are rate limiting
The first 2 cases are illustrated in Figure 1.9.
In the first case, when metabolite disposition is limited by its formation, the half‐life of parent is greater than that of its metabolite and the following equation holds:
(1.51) 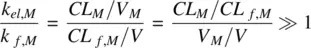
CL Mis the clearance of the metabolite, CL f,Mis the metabolic clearance of the parent drug related to the formation of metabolite, V Mis the volume of distribution of the metabolite, and V is the volume of distribution of the parent drug. The PK profiles of the parent drug and its metabolite are characterized by parallel terminal slopes (see Figure 1.9). The metabolite is eliminated as soon as it is formed. Consequently, the right‐hand side of Equation 1.50becomes zero leading to
(1.52) 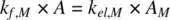
Rearranging Equation 1.52,
(1.53) 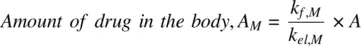
If the parent and metabolite have similar volumes of distribution, according to Equation 1.51, the clearance of the metabolite is greater than that of the parent drug. Thus, the amount of parent drug in the body is always greater than the metabolite. If the volume of distribution of the metabolite is much smaller compared to that of parent drug, then according to Equation 1.51, it is possible that the ratio of the elimination rate constants of the parent and metabolite is ≫1, provided V M /V is smaller than CL M /CL f,M. It is therefore not necessary that CL Mshould be greater than CL f,M. In other words, the amount of parent drug in the body need not be always greater than the metabolite, if the metabolite is polar and has a relatively low volume of distribution compared to the more lipophilic parent drug (see Figure 1.9). An example of this is propranolol where the amount of parent compound is less than that of the metabolite.
In the second case, the metabolite disposition is limited by its elimination and the half‐life of the metabolite is greater than that of its parent drug. This may lead to accumulation of the metabolite in the body and requires careful safety monitoring.
Lastly, in the event of comparable half‐lives of drug and metabolite, the terminal slope of the metabolite appears to decline slower than would be expected from its elimination rate constant, sustained by the parent drug.
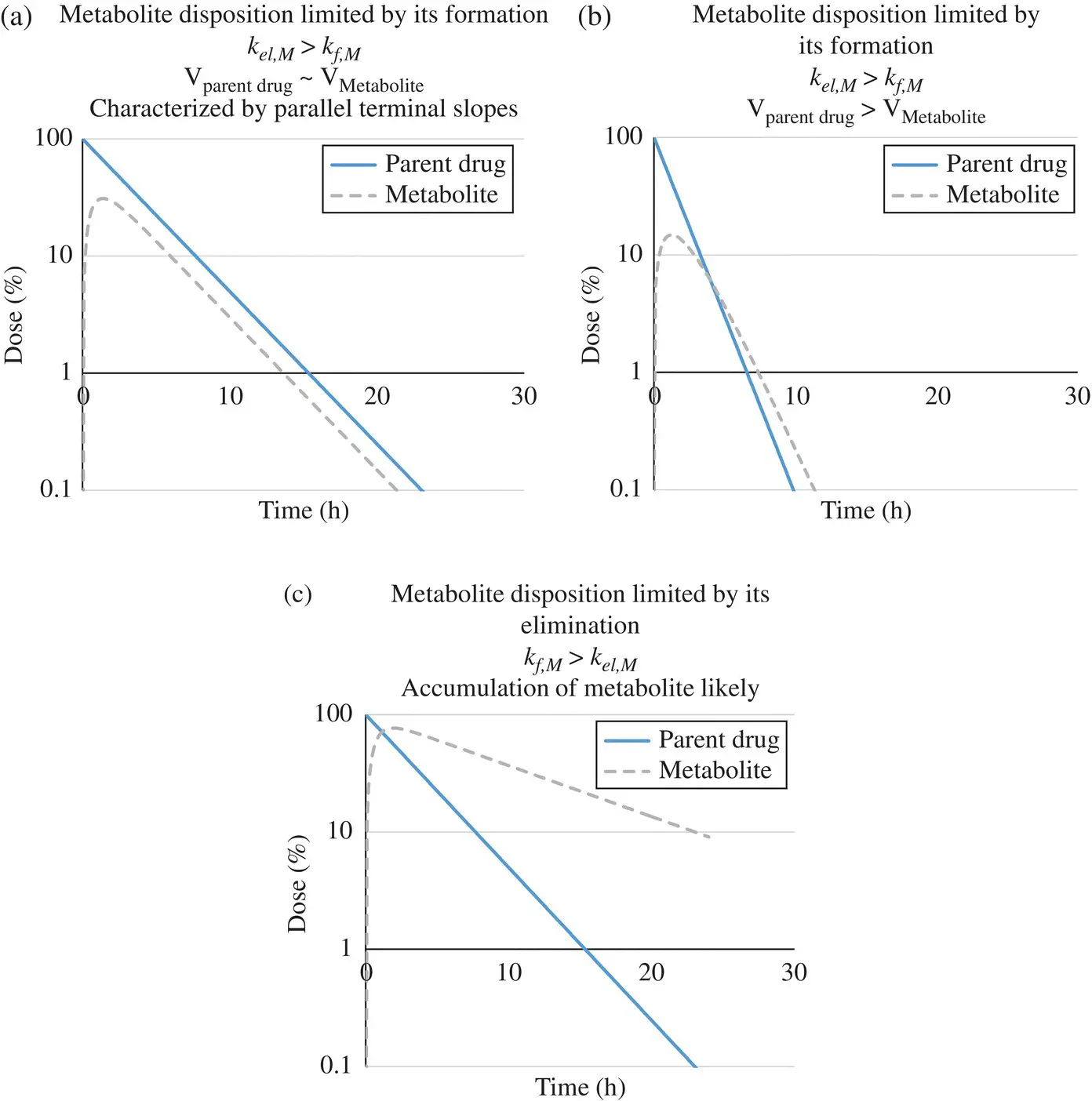
Figure 1.9. Limiting conditions on metabolite disposition. (a) Formation‐limited metabolite kinetics when the volume of distribution of parent is similar to the volume of distribution of its metabolite. (b) Formation‐limited metabolite kinetics when the volume of distribution of parent is greater than the volume of distribution of its metabolite. (c) Elimination‐limited metabolite kinetics.
1.2.10.5 Drug‐Metabolite Plasma Concentration Relationships After Single Drug Administration
Detailed characterization of metabolite PK is limited by the availability of pure standards and chemical stability of metabolites. However, when the metabolite is likely to be active or toxic, the plasma concentration‐time profile of the metabolite is measured. Analogous to Equation 1.50, one can obtain Equation 1.54, recognizing that the product of elimination rate constant and amount of drug is the same as the product of clearance and concentration (see Equation 1.2)
(1.54) 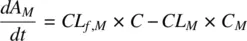
Integrating Equation 1.54,
(1.55) 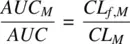
where AUC Mand AUC are the areas under the concentration‐time profiles of metabolite and parent drug, respectively. Recognizing that CL f,Mis the product of fraction metabolized ( f m) and the total clearance ( CL ) of the parent drug,
(1.56) 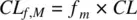
Substituting for CL f,Mfrom Equation 1.56in Equation 1.55,
(1.57) 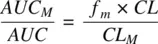
f mis obtained by measuring the amount of metabolite excreted in urine collected over a sufficient length of time (see Equation 1.29).
1.2.10.6 Steady‐State Metabolite Kinetics
At steady state, the rate of change in the amount of metabolite in the body is zero, making the left‐hand sides of Equations 1.50and 1.54equal zero. It follows that the amount and concentration of the metabolite in the body at steady state are:
(1.58) 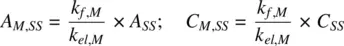
1.2.10.7 First‐Pass Metabolite Production
Following oral drug administration, some drugs have high hepatic and/or gut extraction due to first pass metabolism. The amount of a metabolite of interest in systemic circulation at any given time is the sum of the amounts from the first pass and from the fraction of parent drug escaping first pass ( Figure 1.10).
1.3 PHARMACOKINETIC VARIABILITY
The exposure of a drug in the body is determined by the rate and extent of absorption, distribution, metabolism, and excretion of the drug in an individual, each of which are impacted by drug properties, physiology as well as the activities of enzymes, transporters, and plasma proteins in that individual. Multiple factors impacting these biological parameters ( Table 1.3) include demographics (age, gender, ethnicity, height, and weight), genetic polymorphism, renal or hepatic impairment, obesity, and pregnancy ( Figure 1.11). Thus, the pharmacokinetics of a drug is associated with interindividual variability.
Читать дальше