Mohamed N. Rahaman - Materials for Biomedical Engineering
Здесь есть возможность читать онлайн «Mohamed N. Rahaman - Materials for Biomedical Engineering» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Materials for Biomedical Engineering
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Materials for Biomedical Engineering: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Materials for Biomedical Engineering»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
A comprehensive yet accessible introductory textbook designed for one-semester courses in biomaterials Materials for Biomedical Engineering: Fundamentals and Applications
Materials for Biomedical Engineering: Fundamentals and Applications
Materials for Biomedical Engineering — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Materials for Biomedical Engineering», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
3.3.3 Structure of Inorganic Glasses
The term glass commonly refers to inorganic materials that have an amorphous structure. Although the structure of a glass has short‐range order arising from bonding of an atom with its immediate neighbors, the structure has no long‐range order. Silica can be produced in both crystalline and amorphous states and a comparison of their structures provides a useful illustration of the difference between a crystalline material and a glass ( Figure 3.9). Although silica glass is composed of SiO 4tetrahedra with corner‐sharing oxygen atoms as in crystalline silica, its three‐dimensional structure is composed of a network with no long‐range order.
A problem with silica glass is that it starts to soften only at high temperature (above ~1200 °C), making it difficult to form the glass into objects with desirable shapes. Consequently, metal oxides are commonly added to silica during the production process to obtain a glass that can be formed and shaped more easily. These metal oxides, referred to as network modifiers, add cations to the glass structure and break up a fraction of the Si–O–Si bonds. In quantities of a few percent to a few tens of percent by weight, the network modifiers often include sodium oxide (Na 2O) and calcium oxide (CaO), but a variety of network modifiers are used in many glasses to achieve the desired properties. Figure 3.11illustrates the structure of a glass with one of the simplest compositions, formed by adding Na 2O to silica and referred to as a sodium–silicate glass. Overall, the positive charge of the sodium cations balance the negative charge of the oxygen atoms that are no longer bonded to two neighboring silicon atoms, called non‐bridging oxygen atoms.
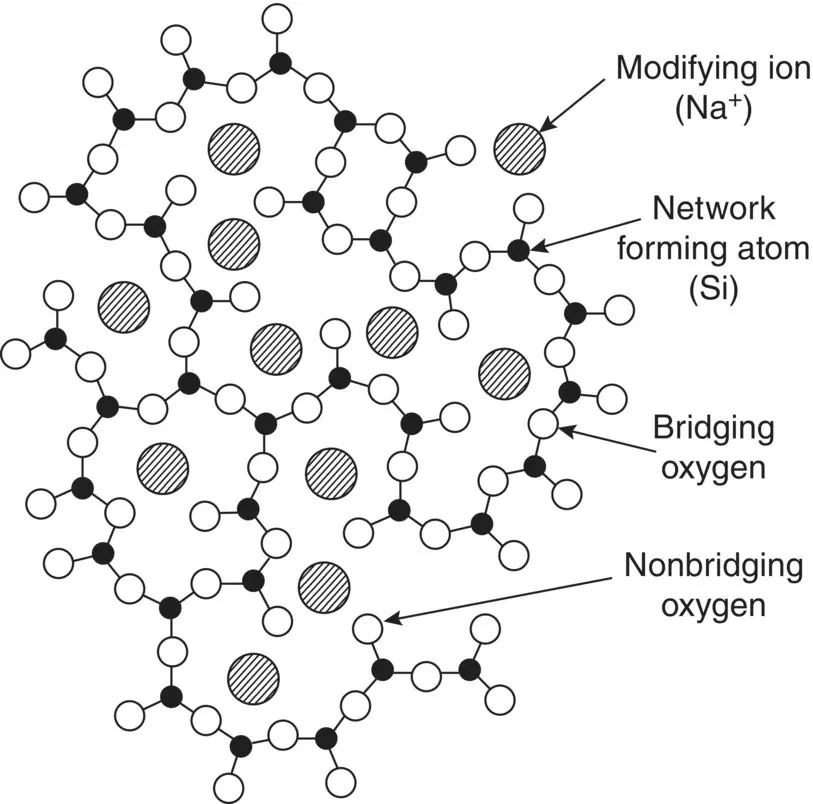
Figure 3.11 Schematic representation of the structure of a sodium silicate glass.
Silicate glasses, composed of the SiO 2glass‐forming network, are the most commonly used glasses, accounting for over ~95% of the tonnage in industrial and commercial applications. A common silicate glass composition is one that is used for windows of buildings, composed of ~70% SiO 2, ~15% Na 2O, and ~10% CaO by weight, plus minor amounts of other oxides. Borate glasses, composed of the B 2O 3glass‐forming network, and borosilicate glasses, composed of interspersed SiO 2and B 2O 3networks, also see considerable use commercially. Phosphate glasses, composed of the P 2O 5glass‐forming network, are used to a more limited extent.
Glasses, nondegradable or bioactive, find limited use as biomaterials, mainly to treat diseased or damaged tissues and organs where their compositional flexibility and ease of forming provide attractive benefits ( Chapter 7). Radioactive glass microspheres, for example, are used to treat liver cancer. Bioactive glasses, such as the silicate glass designated 45S5, are used to a limited extent to heal bone defects while bioactive borate glass in a microfibrous form is used to heal skin wounds.
3.3.4 Structure of Carbon Materials
In the solid state, carbon can exist in several different structures, called allotropes, due to the different ways in which carbon atoms can bond together. Three allotropes, diamond and graphite, both of which are crystalline, and amorphous carbon have been well known for many years. Although they are not compounds, these three carbon materials are often classified as ceramics because they show several properties that are characteristic of covalent‐bonded ceramics. Pyrolytic carbon is an important material in the manufacture of heart valves whereas diamond‐like carbon (DLC) has been investigated as coatings for articulating bearings in hip and knee implants. Diamond and graphite are not used as biomaterials. On the other hand, more recently discovered allotropes of carbon such as fullerenes, carbon nanotubes and graphene, have been the subject of an enormous amount of investigation for use in technological applications and are now receiving considerable interest for potential biomedical applications.
Diamond is often considered prototypical of strongly bonded covalent solids. It consists of a cubic unit cell in which the atoms are located at the corners of a tetrahedron due to sp 3hybridization of the valence electron orbitals of the carbon atoms ( Figure 3.12). While the properties of diamond are very attractive due to its strong covalent bonds, they are more suitable to industrial applications. Diamond is also difficult and expensive to produce industrially.
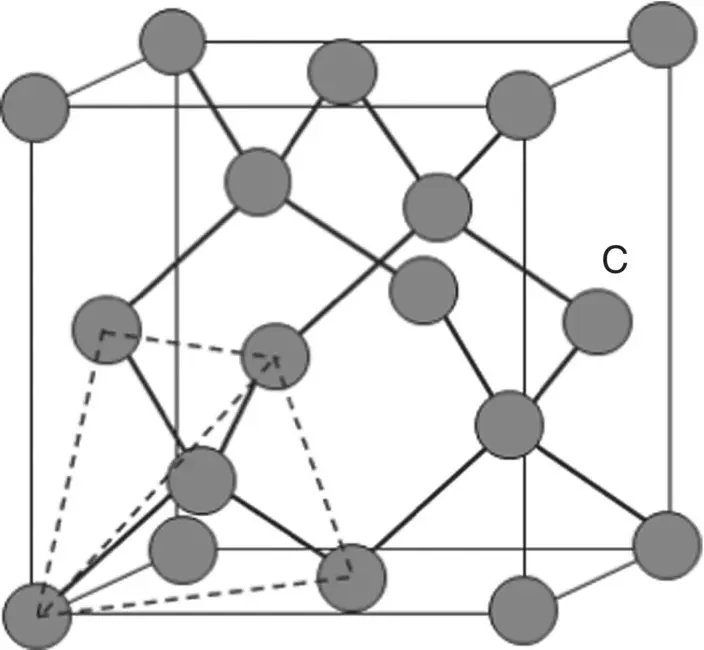
Figure 3.12 Tetrahedral arrangement of covalent bonded carbon atoms in diamond.
Diamond‐like carbon, abbreviated DLC, is a term used to describe a variety of amorphous carbon materials that contain varying amounts of hydrogen. Commonly deposited as a film or coating, DLC has properties that are dependent on the method and carbonaceous precursors used in its production. A high degree of sp 3bonding and a low amount of hydrogen enhance the diamond‐like properties of these materials. Based on their attractive properties such as high hardness, high wear resistance, low coefficient of friction, chemical inertness and biocompatibility, DLC has been investigated for orthopedic applications, such as coatings on articulating metal bearings in hip and knee implants to reduce the amount of wear particles and to improve the corrosion resistance of the metal bearings (Dearnaley and Arps 2005). DLC has also elicited interest for use in cardiovascular applications, such as coatings on stents and heart valves.
DLC coatings for orthopedic applications typically have a high degree of sp 3bonding (approximately 80–85%) and a low amount of hydrogen (less than 1 atomic percent). However, the use of hard coatings on articulating metal bearings has not proved successful in clinical applications due to their unpredictable performance in critical stress conditions. The coating can fracture and chip off when subjected to high local stresses due to wear particles between the articulating surfaces or by inadvertent contact with hard components such as the metal rim of the acetabular cup, resulting in catastrophic wear rates.
Although graphite is not used as a biomaterial, the basic building block of its structure, composed of a planar array of hexagonally arranged carbon atoms ( Figure 3.13), is common to a variety of graphitic materials. In each plane, one s orbital and two p orbitals form three sp 2orbitals that have a trigonal planar arrangement in which each atom is bonded by σ bonds to three neighboring atoms ( Chapter 2). The remaining one electron per carbon atom is delocalized, residing in p orbitals that are perpendicular to the planar arrangement. Neighboring p orbitals overlap, forming π bonds, allowing these delocalized electrons to move more easily within each plane. Thus, each bond in the hexagonal arrangement has partial double bond character. Graphite has a three‐dimensional structure that shows strong covalent bonding within each plane and weak intermolecular bonding between neighboring planes. These structural characteristics are responsible for the well‐known properties of graphite, such as lubricity and electrical conductivity, which are suitable for engineering applications but not relevant for biomedical applications.
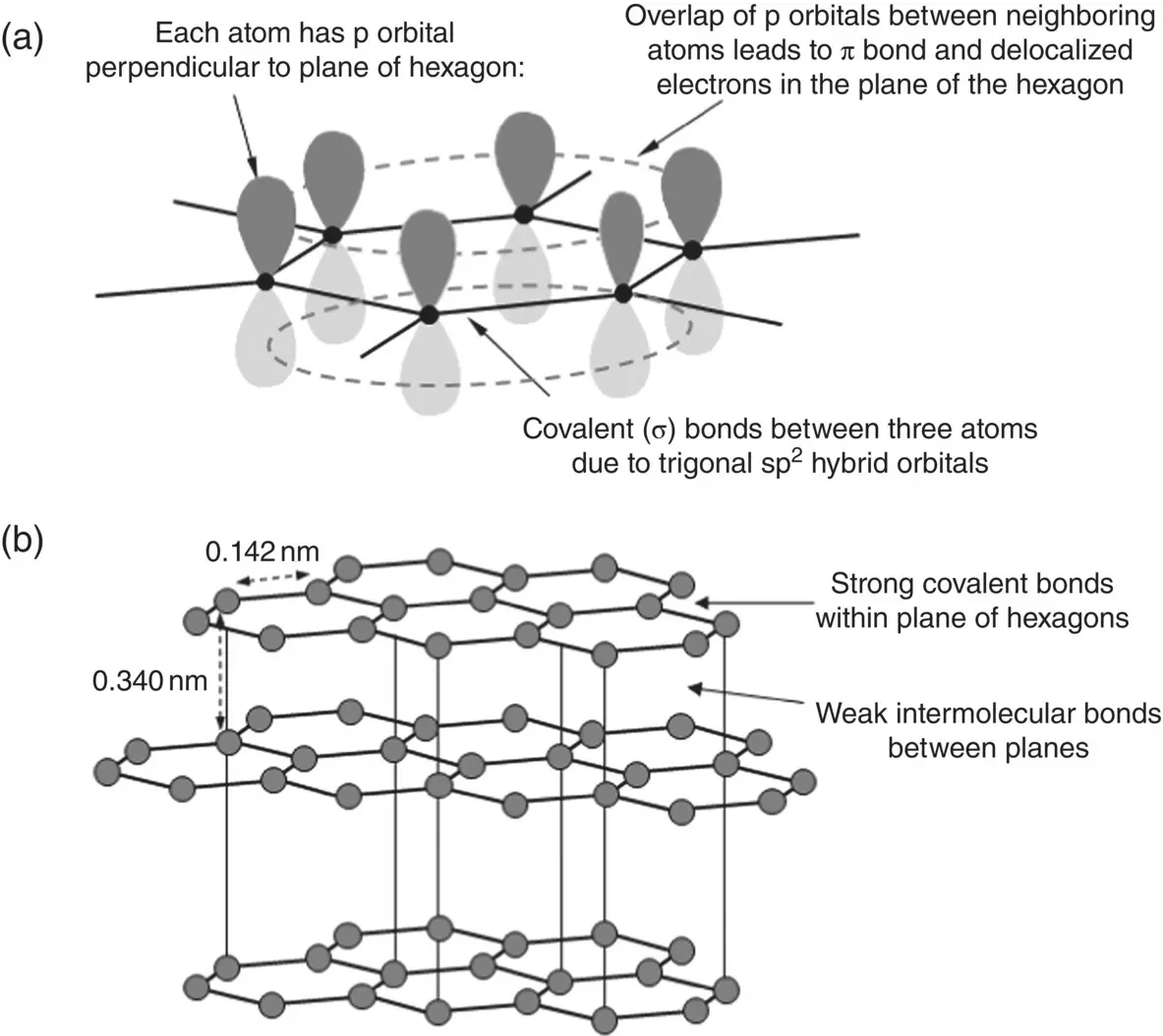
Figure 3.13 (a) Basic building block of graphite composed of a planar array of hexagonally arranged carbon atoms; (b) three‐dimensional arrangement of planar arrays in graphite.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Materials for Biomedical Engineering»
Представляем Вашему вниманию похожие книги на «Materials for Biomedical Engineering» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Materials for Biomedical Engineering» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












