Mohamed N. Rahaman - Materials for Biomedical Engineering
Здесь есть возможность читать онлайн «Mohamed N. Rahaman - Materials for Biomedical Engineering» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Materials for Biomedical Engineering
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Materials for Biomedical Engineering: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Materials for Biomedical Engineering»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
A comprehensive yet accessible introductory textbook designed for one-semester courses in biomaterials Materials for Biomedical Engineering: Fundamentals and Applications
Materials for Biomedical Engineering: Fundamentals and Applications
Materials for Biomedical Engineering — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Materials for Biomedical Engineering», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Pyrolytic carbon is used in the manufacture of mechanical heart valves ( Chapter 24), where it is deposited as a thick coating (~25–500 μm) on graphite to produce valve leaflets (Figure e). In addition to adequate mechanical properties, such as strength, wear resistance, and durability, pyrolytic carbon is highly resistant to blood clotting and causes little damage to blood cells. This combination of properties makes pyrolytic carbon‐coated graphite suitable for use as heart valve leaflets. Pyrolytic carbon belongs to a family of carbon materials called turbostratic carbons that have a similar structure to graphite. However, instead of the flat hexagonal arrays that are stacked and held together by weak interlayer bonding as in graphite, pyrolytic carbon, and other turbostratic carbons are composed of layers that are disordered, resulting in wrinkles or distortions within layers. These disordered layers give pyrolytic carbon improved durability and a smoother surface when compared to graphite.
Fullerenes, Graphenes, and Carbon Nanotubes
Fullerenes, graphenes, and carbon nanotubes share the same basic building block as graphite, a monolayer of hexagonally arranged carbon atoms. Graphene is the name given to a two‐dimensional flat monolayer of carbon atoms arranged in a hexagonal lattice ( Figure 3.14a). The stacking of these flat monolayers gives the three‐dimensional structure of graphite. Graphene is a one‐atom thick, two‐dimensional crystal, often of size less than 1 mm (Geim and Novoselov 2007). The term graphenes is used in a more general sense to describe two‐dimensional crystals composed of one, two or a few (3–10) monolayers that are each distinguishable by their electronic structure. Structures thicker than ~10 monolayers are typically described as thin films of graphite.
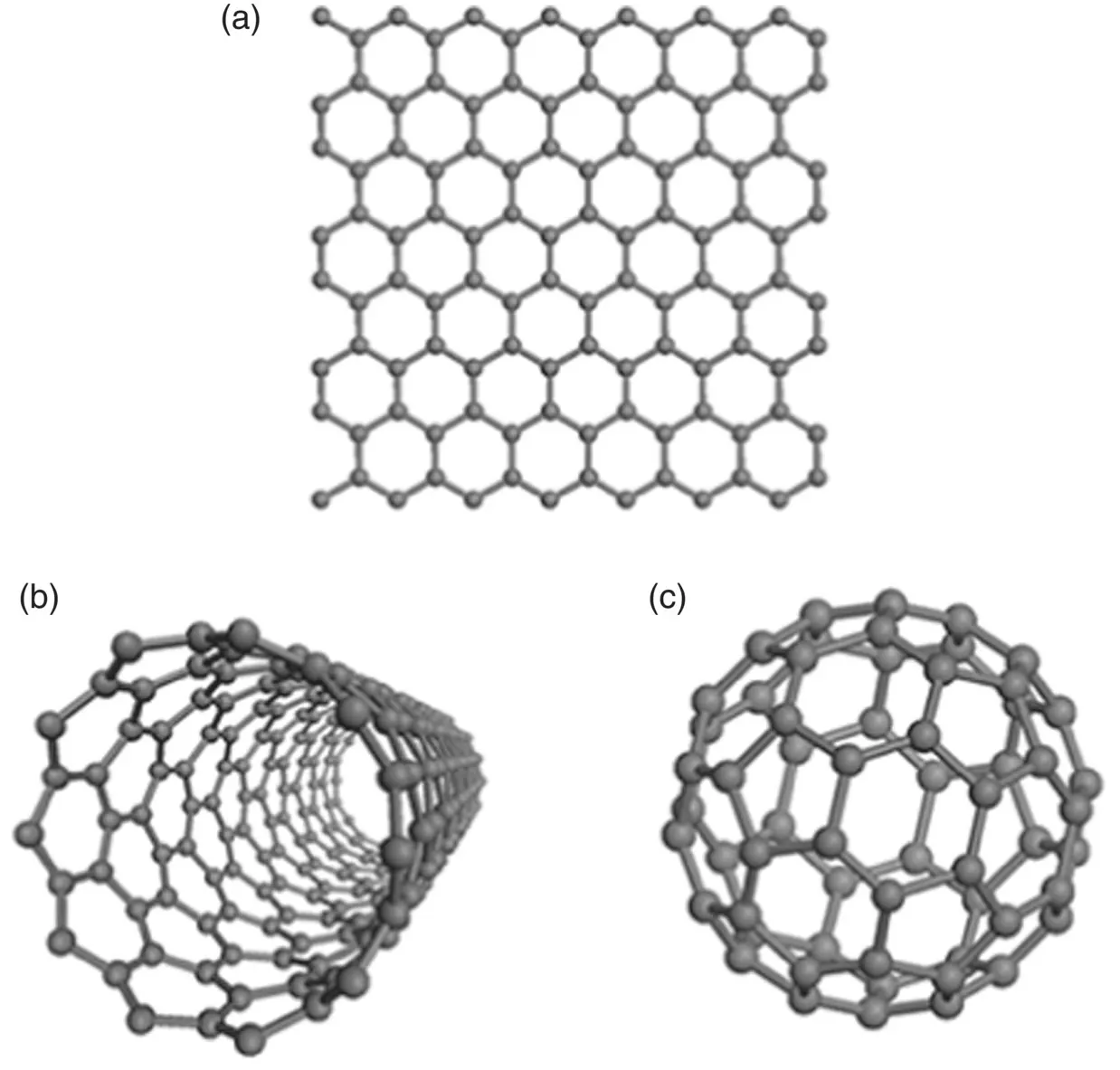
Figure 3.14 Arrangement of carbon atoms in (a) graphene, (b) single‐walled carbon nanotube, and (c) buckminsterfullerene, C 60.
The structure of carbon nanotubes can be viewed as graphene rolled to form a tubular geometry. Carbon nanotubes may consist of single tubes ( Figure 3.14b), called single‐walled carbon nanotubes (SWNTs), or concentric tubes called multi‐walled carbon nanotubes (MWNTs). SWNTs have a diameter from ~1 nm to a few tens of nanometers and lengths of hundreds of nanometers to a few millimeters (Iijima and Ichlhashi 1993). As the long‐range periodicity of the atomic arrangement is retained along the axial direction of the tube, SWNTs may be viewed as one‐dimensional crystals.
Fullerenes are large molecules with a structure that can be viewed as a monolayer composed of hexagonal and pentagonal arrangements of carbon atoms which has been used to form a cage‐like geometry (Giacolone and Martin 2006). The most commonly investigated fullerene is the buckminsterfullerene, C 60, also called a buckyball, composed of 60 carbon atoms joined together to form 20 hexagons and 12 pentagons ( Figure 3.14c). This arrangement of hexagons and pentagons, called a truncated icosahedron, has a pattern similar to a soccer ball. The carbon atoms in the pentagons are bonded by single bonds whereas the bonds in the hexagons consist of resonant double bonds. Although classified as a molecule, the C 60fullerene has a diameter (0.7 nm) close to the lower limit of nanoparticles. When dispersed in a solvent, fullerenes often form aggregates due to weak van der Waals attractive forces, showing properties characteristic of nanoparticles rather than molecules.
The strong covalent (σ) bonds due to the sp 2hybrid orbitals, delocalized electrons of the π bonds due to the overlapping p orbitals in the hexagonally arranged carbon atoms and small size endow graphenes, carbon nanotubes, and fullerenes with unique physico‐chemical properties. These structures have received an enormous amount of scientific and technological interest since their discovery and are now receiving considerable interest for potential biomedical applications, such as drug delivery, gene delivery, biomedical imaging, biosensing, and tissue engineering (Eatemadi et al. 2014; Zhao et al. 2017).
As carbon is chemically inert and hydrophobic, a related area of investigation is the functionalization of graphenes, carbon nanotubes, and fullerenes with appropriate molecules for optimal use in these applications ( Chapter 13). In common with nanostructured materials such as nanoparticles intended for use in vivo , the possible toxicity of graphenes, carbon nanotubes, and fullerenes has been widely studied. While the vast majority of studies have shown no serious adverse response from cells and tissues, some questions still remain about possible toxic effects.
3.3.5 Structure of Polymers
Polymers are composed of long molecules (macromolecules) in which hundreds or thousands of atoms are joined together by covalent bonds to form a chain. The chain backbone in the vast majority of polymers is composed of carbon atoms, as in polyethylene (PE), for example (Figure 2.10), but many polymers have a chain backbone that contains atoms other than carbon, such as silicon, or in addition to carbon, such as silicon, nitrogen, or oxygen. Macromolecules of commonly used degradable polymers such as polylactic acid (PLA), polyglycolic acid (PGA), and polycaprolactone (PCL), for example, contain carbon‐oxygen bonds in addition to carbon–carbon bonds ( Chapter 9).
Intermolecular forces resulting from van der Waals bonding, hydrogen bonding or a combination of these two types of physical bonding are present in all polymers. On the other hand, depending on the chemical composition of the polymer and how it is synthesized, neighboring chains can also be joined chemically at various points, that is, cross‐linked by covalent bonds. The elasticity of synthetic rubbers used in engineering applications and elastin, a component of skin and other tissues, is due to the presence of chemical crosslinks between the molecules. Macromolecules in the vast majority of polymers are also more complex than the simple linear structure of PE ( Chapter 8). Instead of just hydrogen atoms bonded to carbon atoms in the chain backbone, the side groups can have a variety of composition, structure and size, and they can be bonded to carbon atoms of the chain backbone at regular or random intervals. These factors influence the way in which the macromolecules pack together in a solid.
In the majority of solid polymers, when the macromolecules pack together they do so in a random manner, giving an amorphous structure ( Figure 3.15a). In other polymers, commonly those in which the macromolecules have a rather simple structure with little or no crosslinking, the chains can pack in an ordered pattern by folding backward and forward ( Figure 3.15b). This regular repeating pattern of chain folding leads to the formation of crystalline regions. As long as the macromolecular structure allows this type of ordered arrangement, the formation of crystalline regions is possible because there is a driving force for it. This is because a crystal has a lower energy than an amorphous structure of the same composition due to the ordered and closer packing of the polymer chains. However, it is difficult for a macromolecule to pack completely into a crystalline pattern even for the simplest structure. Consequently, these polymers are composed of crystalline regions interspersed with amorphous regions ( Figure 3.15b). The term semicrystalline has often been used to describe these polymers, in a sense to indicate that they are not totally composed of crystals. Generally, formation of crystalline regions is more favorable when the side groups are less bulky, are bonded at regular intervals to the chain backbone, or allow substantial intermolecular bonding such as hydrogen bonding to stabilize the ordered arrangement of the macromolecules.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Materials for Biomedical Engineering»
Представляем Вашему вниманию похожие книги на «Materials for Biomedical Engineering» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Materials for Biomedical Engineering» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












