Mohamed N. Rahaman - Materials for Biomedical Engineering
Здесь есть возможность читать онлайн «Mohamed N. Rahaman - Materials for Biomedical Engineering» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Materials for Biomedical Engineering
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Materials for Biomedical Engineering: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Materials for Biomedical Engineering»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
A comprehensive yet accessible introductory textbook designed for one-semester courses in biomaterials Materials for Biomedical Engineering: Fundamentals and Applications
Materials for Biomedical Engineering: Fundamentals and Applications
Materials for Biomedical Engineering — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Materials for Biomedical Engineering», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Although it is not used as a biomaterial, NaCl is often taken as an example of a ceramic material that shows a high degree of ionic bonding. In NaCl crystals, each type of ion is surrounded, that is, coordinated, by six ions of the opposite sign. ( Figure 3.8). The unit cell is cubic and the structure can be considered as composed of two interpenetrating FCC lattices of the cations (Na +) and anions (Cl −).
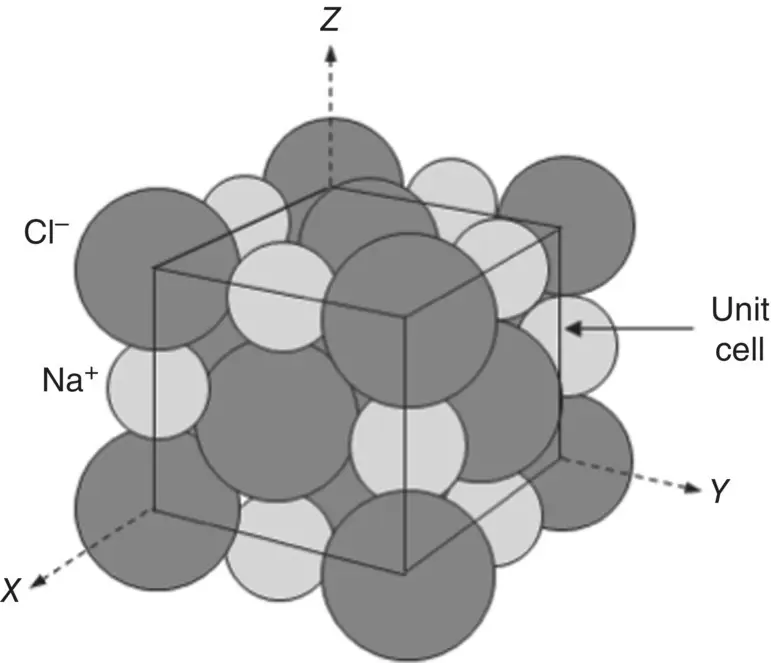
Figure 3.8 Arrangement of sodium ions (Na +) and chlorine ions (Cl −) in a sodium chloride unit cell.
Aluminum oxide (Al 2O 3), often referred to as alumina or α‐alumina, is used as articulating bearings in hip implants ( Chapter 1). Although alumina has aclose‐packed hexagonal (CPH) structure, the arrangement of the ions has to take into account the dissimilar cation to anion ratio (2 : 3) and the geometrical restriction that each type of ions must not touch. The structure can be viewed as composed of layers of O 2−ions stacked in a direction perpendicular to the basal plane between which two‐thirds of the interstitial atomic sites are occupied by Al 3+ions and one‐third is empty.
Zirconium oxide (ZrO 2), often referred to as zirconia, exists in three different crystalline forms called polymorphs ( Chapter 7). The cubic form transforms to tetragonal below ~2370 °C that, in turn, transforms to a monoclinic structure below ~1240 °C. In addition to the change in crystal structure, the transformation to the monoclinic structure is accompanied by a volume expansion of ~4.7% that cannot be easily accommodated in the solid. When zirconia is cooled from its fabrication temperature, typically well above the tetragonal to monoclinic transition temperature, this volume change leads the creation of microcracks and, thus, to a weak material. Consequently, zirconia is not useful in engineering or biomedical applications. On the other hand, upon the addition of a few percent of yttrium oxide (yttria), Y 2O 3, or magnesium oxide (magnesia), MgO, for example, the cubic or tetragonal phase can be retained as a metastable phase on cooling to room temperature. When fabricated under well‐controlled and reproducible conditions, yttria‐stabilized zirconia (YSZ) with a tetragonal structure (~3 mol% Y 2O 3), is among the ceramics with the best mechanical properties. YSZ is shaped and used as dental restorations such as crowns and bridges and, in particulate form, it is incorporated into alumina in the production of zirconia‐toughened alumina (ZTA) bearings for hip implants. These bearings have better resistance to cracking than alumina due to transformation of the YSZ particles to the monoclinic phase in the vicinity of an approaching crack ( Chapter 7). As this transformation uses up some of the energy that would be otherwise available for crack propagation, growth of the crack is blunted.
Many of the strongest, hardest, and most refractory ceramics have structures with a high degree of covalent bonding. Several of these ceramics, such as carbon in the form of diamond, silicon carbide (SiC) and silicon nitride (Si 3N 4), for example, show a tetrahedral arrangement of the bonds due to sp 3hybridization of the valence electron orbitals in atoms such as carbon and silicon ( Chapter 2). As there are a fixed number of directional bonds in covalent bonding, the position and number of neighboring atoms in the structure are also fixed. Diamond is often considered prototypical of solids that show strong covalent bonds. It consists of a cubic unit cell in which the atoms are located at the corners of a tetrahedron due to sp 3hybridization of the valence electron orbitals of the carbon atoms ( Section 3.3.4). Silicon nitride is used in spinal fusion implants and it is being developed for use as femoral head bearings in hip implants. It has a hexagonal crystal structure in which each silicon atom is bonded to four nitrogen atoms due to sp 3hybridization of the silicon atom. These SiN 4tetrahedra are joined together by three nitrogen atoms in a trigonal planar arrangement due to sp 2hybridization of the electron orbitals in nitrogen.
In silicon dioxide (SiO 2) commonly called silica, the four hybridized sp 3orbitals in silicon leads to a strong preference for the formation of SiO 4tetrahedra, in which the oxygen atoms are located at the corners of the tetrahedron in both the crystalline and amorphous states. Crystalline silica, for example, is composed of an ordered repeating pattern of three‐dimensional structures in which the oxygen atom at each corner of an SiO 4tetrahedron is shared with that of a neighboring tetrahedron. Crystalline silica occurs in three different crystalline forms (polymorphs) called cristobalite, tridymite, and quartz. Figure 3.9a illustrates the arrangement of the SiO 4tetrahedra in cristobalite.
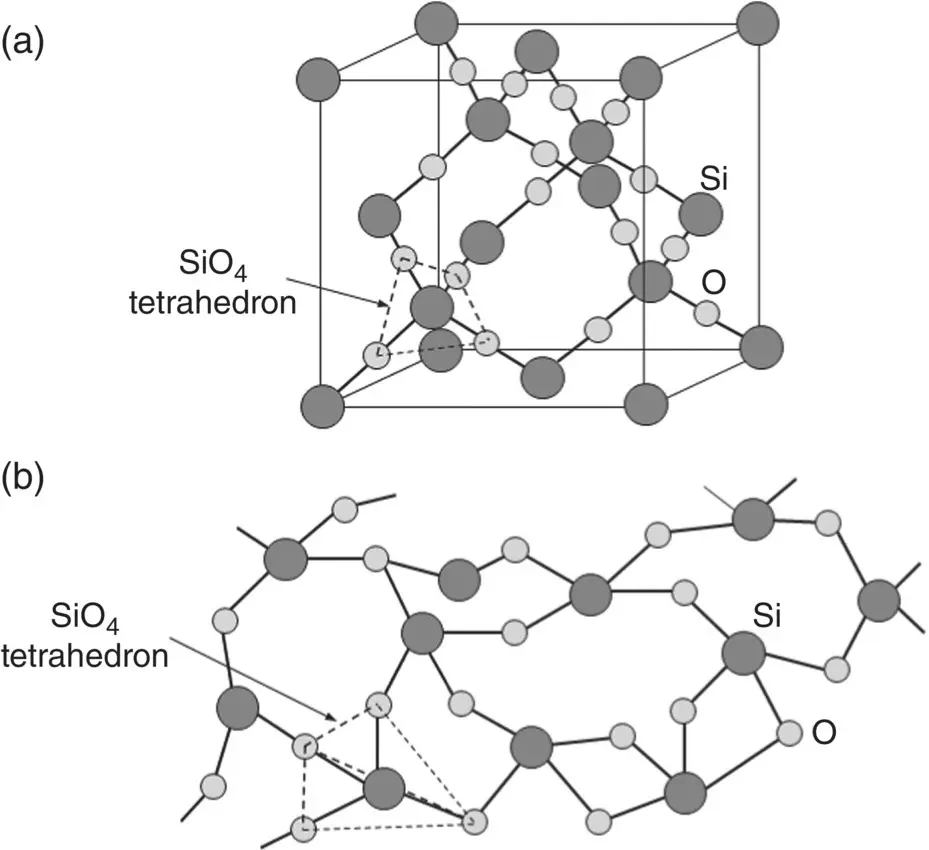
Figure 3.9 Illustration of (a) ordered arrangement of SiO 4tetrahedra in crystalline silica (cristobalite) and (b) non‐ordered arrangement in amorphous silica glass.
Crystal Structure of Hydroxyapatite
Several types of synthetic calcium phosphate materials have been studied or developed for use in biomedical applications such as bone reconstruction, drug delivery devices and coatings for metal prostheses ( Chapter 7). Implants composed of hydroxyapatite, with the formula Ca 10(PO 4) 6(OH) 2, β‐tricalcium phosphate, Ca 3(PO 4) 2, and a two‐phase mixture called biphasic calcium phosphate (BCP), composed of varying ratios of hydroxyapatite and β‐tricalcium phosphate, for example, have been used for decades in healing bone defects. The primary building block of β‐tricalcium phosphate and hydroxyapatite is the phosphate ion (PO 4) 3−in which the phosphorus and oxygen atoms form a slightly distorted tetrahedron ( Figure 3.10a). As the main inorganic (mineral) constituent of hard tissues such as bone and teeth is composed of a material that resembles hydroxyapatite, considerable attention has been devoted to the structure and properties of this material. Hydroxyapatite exists in a monoclinic or a hexagonal crystal structure. The unit cell in the hexagonal structure has dimensions a = b = 0.9430 nm and c = 0.6891 nm, and bond angles α = β = 90° and γ = 120°, and it is composed of 6 (PO 4) 3−ions surrounded by 10 calcium ions and with 2 hydroxyl (OH) −ions along the c‐ axis ( Figure 3.10b).
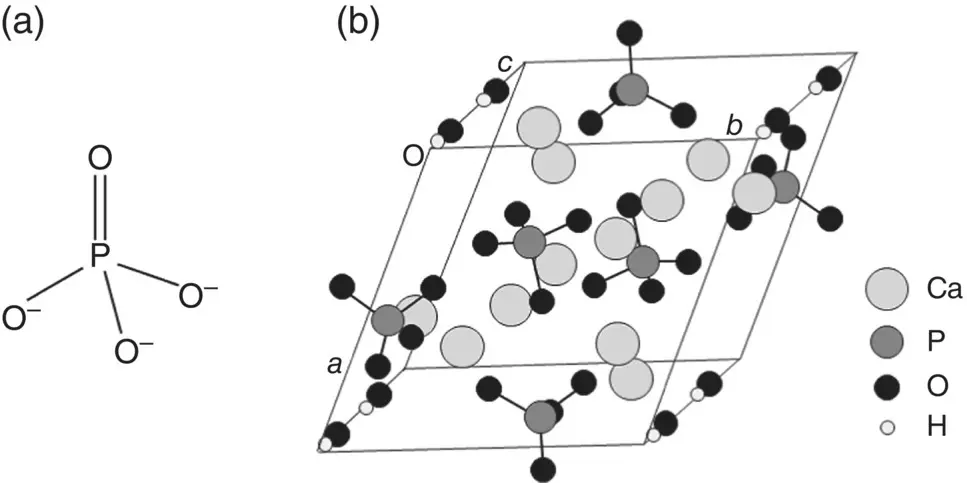
Figure 3.10 (a) Arrangement of atoms in a phosphate (PO 4) 3−ion; (b) arrangement of atoms (ions) in unit cell of hydroxyapatite.
The name hydroxyapatite refers to a crystalline material with the stoichiometric composition Ca 10(PO 4) 6(OH) 2. In comparison, the inorganic constituent of hard tissues such as bone and teeth is not pure hydroxyapatite. Instead, it has a composition that deviates from this stoichiometric composition to varying extent, depending on the living organism and the location of the hard tissue in the organism. The host ions in hydroxyapatite are substituted by small to trace amounts of other ions, such as (CO 3) 2−, (HPO 4) 2−, Mg 2+, Na +, Sr 2+, K +, and F −( Section 3.4.1). This substituted composition, is one factor that contributes to the unique properties of the hydroxyapatite‐like material of bone. As the properties of a material are dependent on its composition, there has been significant interest in producing synthetic hydroxyapatite‐like materials with compositions that approximate the inorganic constituent of bone ( Chapter 7).
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Materials for Biomedical Engineering»
Представляем Вашему вниманию похожие книги на «Materials for Biomedical Engineering» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Materials for Biomedical Engineering» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












