Mohamed N. Rahaman - Materials for Biomedical Engineering
Здесь есть возможность читать онлайн «Mohamed N. Rahaman - Materials for Biomedical Engineering» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Materials for Biomedical Engineering
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Materials for Biomedical Engineering: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Materials for Biomedical Engineering»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
A comprehensive yet accessible introductory textbook designed for one-semester courses in biomaterials Materials for Biomedical Engineering: Fundamentals and Applications
Materials for Biomedical Engineering: Fundamentals and Applications
Materials for Biomedical Engineering — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Materials for Biomedical Engineering», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
2.6.4 Quaternary Structure
Some globular proteins, often called oligomeric proteins, are composed of two or more different polypeptide chains. The quaternary structure of these proteins emphasizes the way in which the individual folded protein chains fit together to form an overall three‐dimensional structure ( Figure 2.27). A well‐studied example is hemoglobin, the protein that carries oxygen in red blood cells. This protein has a nearly spherical shape, composed of two identical α‐globin subunits and two identical β‐globin subunits arranged symmetrically. The same noncovalent bonds that stabilize the tertiary structure stabilize the quaternary structure of proteins.
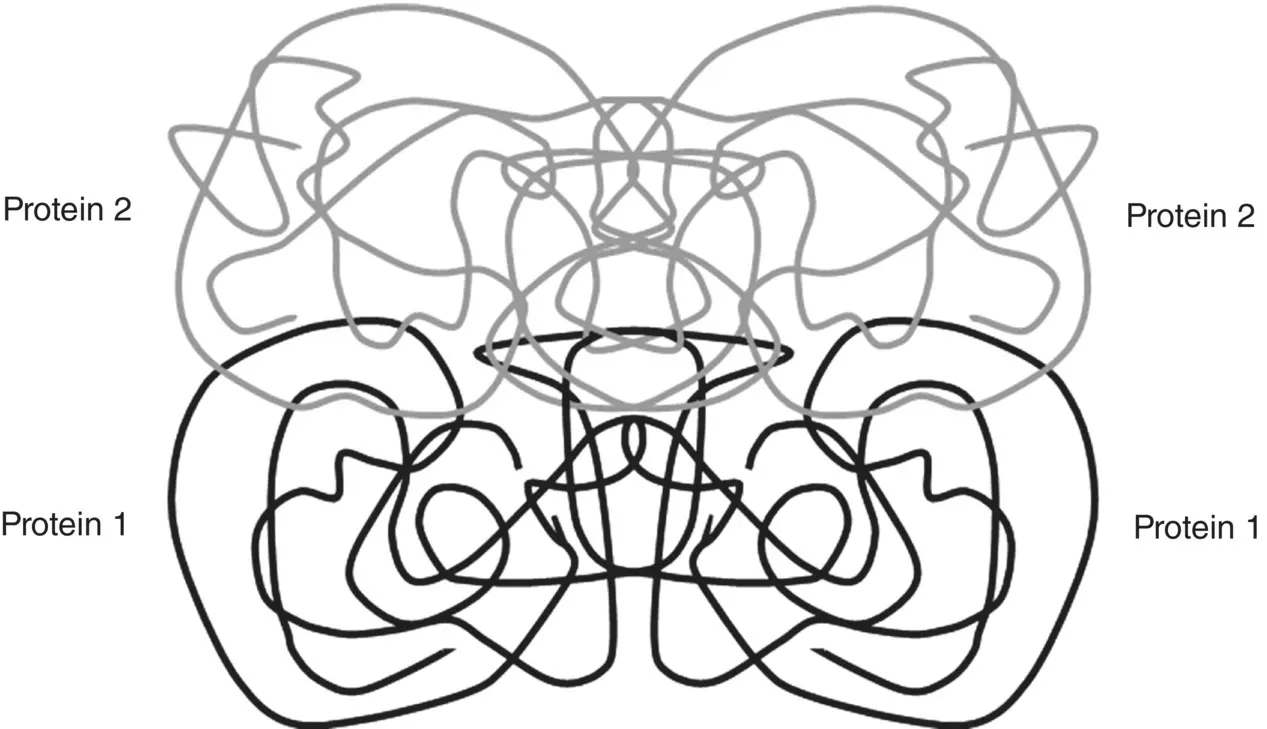
Figure 2.27 Illustrative example of quaternary structure of a protein composed of four polypeptide chains. In this example, there are two types of protein, labeled Protein 1 and Protein 2, each with two chains.
Table 2.6summarizes the four levels of protein structure and the main atomic interactions.
Table 2.6 Summary of the four levels of protein structure and major atomic interactions that stabilize each level.
| Structure | Description | Geometry | Major atomic interactions |
|---|---|---|---|
| Primary | Sequence of amino acid residues in chain backbone | — | Covalent bonding between amino acid residues to form amide (peptide bond) |
| Secondary | Arrangement of chain backbone of individual polypeptide | Random arrangementRegular helical structure (α‐helix)Regular sheet structure (β‐sheet) | Intrachain hydrogen bonding |
| Tertiary | Three‐dimensional shape (conformation) of polypeptide chain | GlobularFibrous (elongated) | Interchain and intrachain hydrogen, van der Waals, ionic, and covalent bonding a |
| Quaternary | Overall three‐dimensional structure of oligomeric proteins | Approximately spherical or irregular | Interchain hydrogen and van der Waals |
aHydrogen and van der Waals bonding often dominate over ionic and covalent bonding.
2.7 Concluding Remarks
Bonding between atoms and molecules and the way in which the atoms or molecules pack together, whether in an ordered crystalline structure or a disordered amorphous structure, are crucial in understanding the intrinsic properties of materials. In this chapter, we discussed bonding between atoms and molecules, which can be divided into two types: strong primary (interatomic) bonds between atoms and weak secondary (intermolecular) bonds between molecules.
Ionic and covalent bonds, common in ceramics, and metallic bonding, common in metals and metal alloys, are the three types of primary bonds. Polymers have strong interatomic covalent bonds between atoms in the chain backbone but weak intermolecular bonding between the chains.
We discussed representations of the interaction between two atoms, in terms of a force or potential energy as a function of interatomic distance, which provide a useful way to understand how many of the intrinsic properties of a material arise
Van der Waals and hydrogen bonds are the main types of intermolecular bonding
Proteins, the most versatile macromolecules in living organisms, have a complex three‐dimensional shape (conformation) that is determined by intrachain and interchain covalent and noncovalent bonds. We discussed how the bonding in proteins determines their overall three‐dimensional shape, which, in turn, has a critical effect on their functionality.
Problems
1 2.1 Predict the type of bond (ionic, covalent, metallic, or a combination of these) that will be present in each of the following molecules. Explain your answer.NaFCH4Al2O3SiCCHCl3
2 2.2 Distinguish between intrinsic properties of materials and their engineering properties, indicating how these properties are dependent on the structure of a material.
3 2.3 Given the ionic radii of the following ions: sodium = 0.102 nm; chlorine = 0.181 nm; magnesium = 0.072 nm; oxygen = 0.140 nm, determine the potential energy of attraction in (kJ/mol) for (i) the NaCl molecule, and (ii) the MgO molecule at a separation distance equal to the sum of the ionic radii. Comment on the difference between these values and the bonding energy in Table 2.2for these molecules.
4 2.4 Sodium chloride (NaCl) and silicon dioxide (SiO2) are respectively among the prototype ionic and covalent solids. The electronegativities of the atoms in the Pauling electronegativity scale are as follows: sodium = 0.93; chlorine = 3.16; silicon = 1.90; oxygen = 3.44. Determine the percentage ionic and covalent character of the bonds in NaCl and SiO2 according to Eq. (2.5).
5 2.5 Carbon and silicon both have four electrons in their outermost shell but carbon dioxide is a gas at room temperature whereas silicon dioxide is a solid with a high melting point. Explain.
6 2.6 Explain why hydrogen fluoride (HF) has a higher boiling point (19.4 °C) than that (−85 °C) of hydrogen chloride (HCl) even though its molecular weight is lower.
7 2.7 The trichloromethane (CHCl3) molecule is polar whereas the tetrachloromethane molecule is nonpolar but, despite its polarity, trichloromethane has a boiling point (61.2 °C) that is lower than that of tetrachloromethane (76.7 °C). Explain.
8 2.8 The pKa values of the side groups in the amino acids glutamic acid, arginine and histidine are 4.3, 12.5, and 6.0, respectively. With the aid of a sketch of each amino acid residue, explain whether the side group will have a positive or negative charge at the physiological pH, and indicate the position of the charge on the side group.
Reference
1 Alberts, B., Johnson, A.D., Lewis, J. et al. (2014). Molecular Biology of the Cell, 6e. New York: W W Norton & Company Chapter 3.
Further Reading
1 Callister, W.D. (2007). Materials Science and Engineering: An Introduction, 7e. New York: Wiley Chapter 2.
2 Ebbing, D.D., Gammon, S.D., and Ragsdale, R.O. (2006). Essentials of General Chemistry, 2e. Boston, MA: Houghton Mifflin Company Chapter 9.
3 Shoulders, M.D. and Raines, R.T. (2009). Collagen structure and stability. Annual Review of Biochemistry 78: 929–958.
3 Structure of Solids
3.1 Introduction
Biomaterials are typically three‐dimensional solids composed of an enormous number of atoms. A titanium (Ti) implant of volume 1 cm 3, for example, contains ~5 × 10 22atoms. Consequently, we are concerned with the structural characteristics at different levels or length scales. Bonding between atoms to form molecules and the way in which the atoms pack together to form a controllable structure determine the intrinsic properties of a solid ( Chapter 2). If the atoms pack together in a regularly repeating three‐dimensional pattern, the structure is described as crystalline. On the other hand, if the atoms pack together in a random non‐ordered pattern, the structure is said to be amorphous. Metals and ceramics in their pure state have a crystalline structure. On the other hand, when cooled at a rapid enough rate from the molten state, some metals and ceramics develop an amorphous structure and the solid is said to be a glass or to have a glassy structure. Whereas glasses are, by definition, amorphous, some materials derived from glasses, called glass‐ceramics, consist of a mixture of crystalline and amorphous phases. Many polymers have an amorphous structure but some have a structure composed of a combination of crystalline and amorphous regions, sometimes described as a semicrystalline structure. Whether a material is crystalline or not is important because a crystalline phase has properties that are different and often better than an amorphous phase of the same composition.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Materials for Biomedical Engineering»
Представляем Вашему вниманию похожие книги на «Materials for Biomedical Engineering» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Materials for Biomedical Engineering» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












