Mohamed N. Rahaman - Materials for Biomedical Engineering
Здесь есть возможность читать онлайн «Mohamed N. Rahaman - Materials for Biomedical Engineering» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Materials for Biomedical Engineering
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Materials for Biomedical Engineering: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Materials for Biomedical Engineering»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
A comprehensive yet accessible introductory textbook designed for one-semester courses in biomaterials Materials for Biomedical Engineering: Fundamentals and Applications
Materials for Biomedical Engineering: Fundamentals and Applications
Materials for Biomedical Engineering — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Materials for Biomedical Engineering», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
In general, the higher the p K avalue, the weaker the acid.
Table 2.4gives the p K avalues of the molecules in the acidic or basic side groups. As the p K avalue for the side group of aspartic acid is ~3.9, the dissociated side groups (C=O)O −have a negative charge at the physiological pH. On the other hand, the p K avalue for the side group of lysine is ~10.5 and, thus, the protonated side groups (N +H 3) in this amino acid have a positive charge at the physiological pH.
Hydrogen bonds: The amide bonds in the protein chain backbone contain polar C=O and N–H bonds while the side group of some amino acid residues contain these bonds or polar hydroxyl (O–H) bonds. These polar species give rise to hydrogen bonding which can again be intrachain or interchain ( Figure 2.23c). Although far weaker than the covalent and ionic bonds, hydrogen bonds are numerous in proteins and, thus, have a significant influence on the conformation of proteins.
Van der Waals bonds: These weak bonds involve electrostatic interactions between polar species in the side groups that do not take part in hydrogen bonding due to a lack of lone pair electrons in the relevant atoms or between nonpolar side groups ( Figure 2.23d).
In an aqueous physiological environment, van der Waals bonding involving the side groups plays an additional role in determining the conformation of proteins. In general, nonpolar molecules are hydrophobic whereas polar and ionic molecules are hydrophilic. Consequently, as the protein chain folds to form a globular three‐dimensional shape, it does so in a manner such that side groups with polar or ionizable molecules gather on the outside of the protein chain backbone whereas side groups with nonpolar molecules are buried on the inside of the chain ( Figure 2.24). This manner of chain folding enhances the interaction of the polar or ionizable side groups with the polar water molecules and shields the nonpolar side groups from the aqueous environment. The nonpolar groups cluster together by van der Waals attraction and this, together with the interactions of the polar and ionizable side groups with the water molecules, has a strong influence on the overall three‐dimensional shape of the protein. The protein undergoes conformational changes until the sum of its interaction energy is minimized.
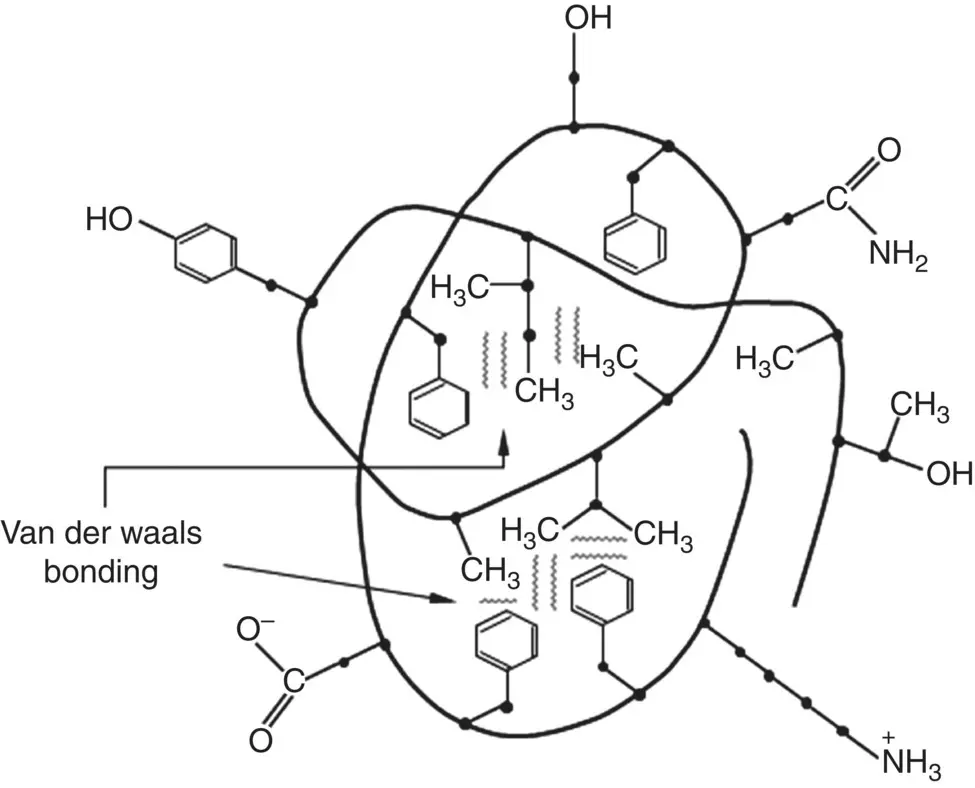
Figure 2.24 Illustration of chain folding of protein to form a globular three‐dimensional shape in an aqueous environment. Side groups with polar or ionizable molecules gather on the outside of the protein chain backbone whereas side groups with nonpolar molecules are buried on the inside of the chain. Some side groups are shown as examples.
In nonaqueous environments, such as the lipid bilayer of the cell membrane, the arrangement of the polar and nonpolar groups is opposite to that in aqueous environments. The nonpolar groups predominantly gather on the outside of the protein chain where they can interact with the nonpolar (hydrophobic) side chains of membrane fatty acids whereas the polar groups arrange themselves anywhere they can contact water. For transmembrane proteins, for example, which project through both sides of the membrane, the polar groups are found at each point where the polypeptide chain emerges from the membrane.
Fibrous Proteins
Fibrous proteins are abundant in mammalian tissues, particularly in the extracellular matrix, a complex structural network of proteins and other substances surrounding and supporting cells. Extracellular matrix proteins are secreted by cells into their surroundings where they often assemble into sheets or fibers. The most abundant of these proteins is a family called collagen. A major component of skin and bone, collagen is the most abundant protein in mammals, comprising approximately 25% of the total dry protein mass.
Collagen is structurally distinct from other proteins in that the molecule consists of three polypeptide chains, called α‐chains, that wind around each other to form a long triple‐helix structure ( Figure 2.25). Glycine, the smallest amino acid, occurs at every third position in the chain backbone. This small size and regular repeating pattern of glycine allows the three chains to wind around each other closely to generate a compact triple‐helix structure. Procollagen molecules secreted from cells are cleaved at their ends to form collagen molecules, each ~1.5 nm in diameter and ~300 nm in length. Side‐to‐side and end‐to‐end bonding between many of these collagen molecules lead to the formation of much larger fibrils that, in turn, can then assemble to form fibers ( Chapter 10). This fibrous morphology is a significant factor in endowing tissues such as tendons and ligaments with their high tensile strength and elastic modulus, in a manner similar to aligned macromolecules that contribute to the high mechanical properties of synthetic fibers such as nylon and polyethylene.
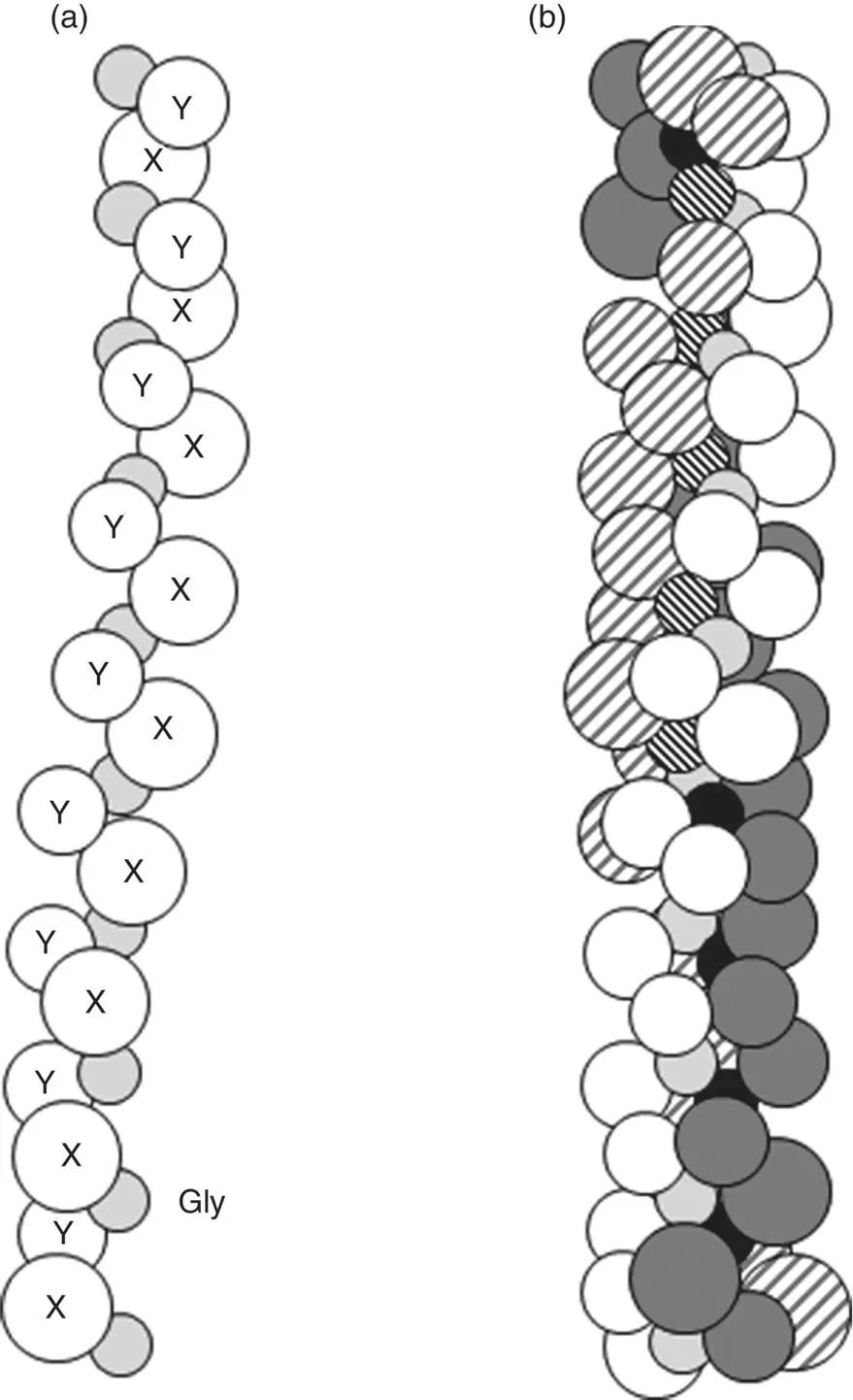
Figure 2.25 (a) Illustration of single α‐chain composed of the amino acid sequence Gly‐X‐Y where Gly is the three letter symbol for glycine that occurs at every third position in the sequence, and X and Y are the one‐letter symbol of any amino acid. (b) Illustration of triple‐helix structure of collagen formed by winding of three α‐chains around each other.
As glycine (Gly) occurs at every third position, the primary structure of each chain has the sequence Gly‐X‐Y, where X and Y represent the one‐letter symbol of the other amino acid residues. X and Y can be any amino acid but, often, they consist of the amino acids proline (Pro) and hydroxyproline (three‐letter symbol Hyp). Typically, approximately one‐third of the amino acid residues in the chain consists of glycine and approximately one‐quarter consists of a combination proline and hydroxyproline, as exemplified by the amino acid content of human tendon ( Table 2.5 ). Hydroxyproline and another modified amino acid, hydroxylysine (three‐letter symbol Hyl), which often occur in proteins, are formed by enzymatic reactions involving proline and lysine residues in the chain prior to triple‐helix formation within the cell ( Figure 2.26).
Table 2.5 Measured amino acid content of human tendon.
| Amino acid | Number of residues per 1000 residues |
|---|---|
| Glycine | 334 |
| Proline | 122 |
| Hydroxyproline | 96 |
| Acidic or polar (Asn; Glu; Asn) | 124 |
| Basic or polar (Lys; Arg; His) | 91 |
| Others | 233 |
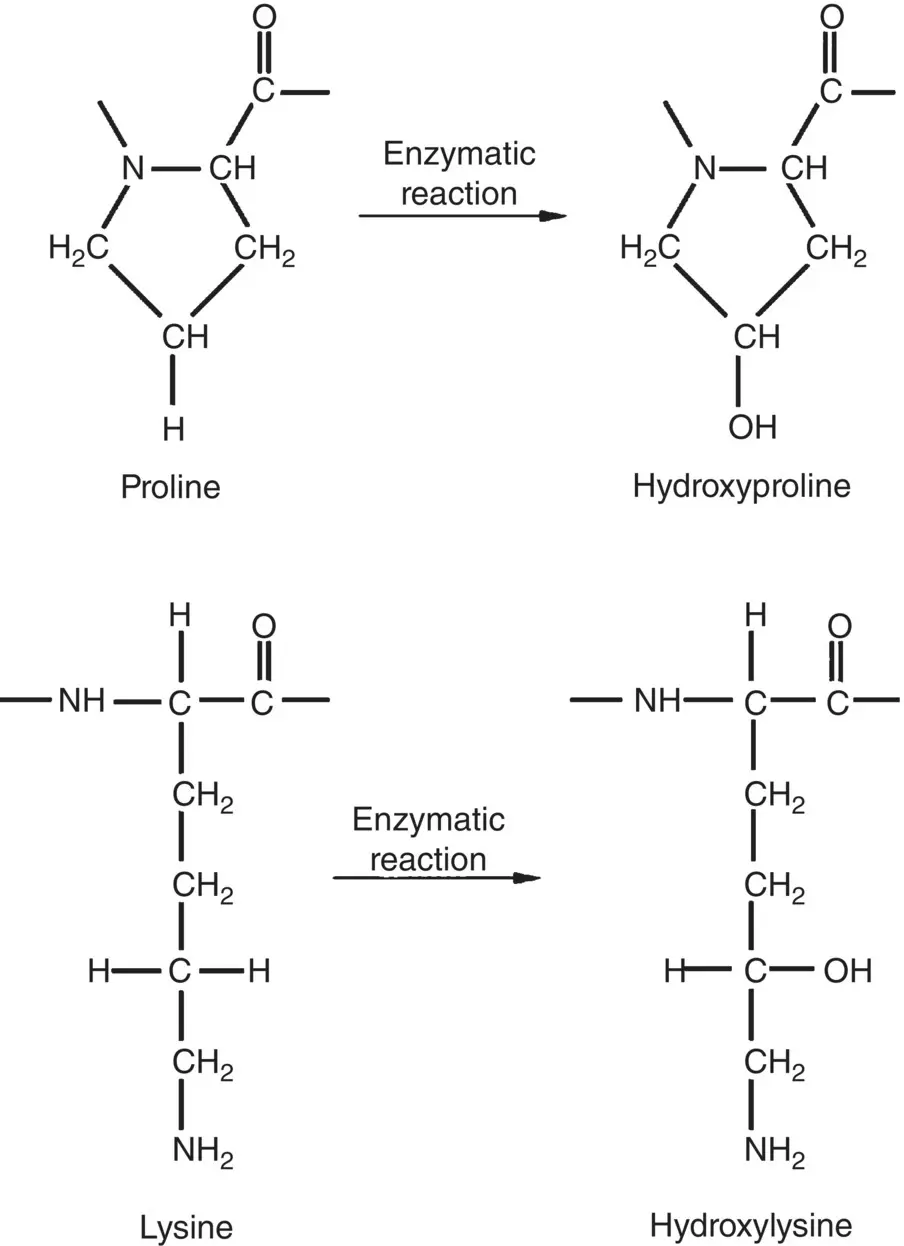
Figure 2.26 Schematic illustration of the structure of the modified amino acid residues hydroxyproline and hydroxylysine formed from proline and lysine residues by enzymatic reaction.
Interchain hydrogen bonding between the carbonyl oxygen (C=O) and amine hydrogen (N–H) stabilizes the triple‐helix structure. Proline also plays a role in stabilizing the structure because it has a limited ability to change its conformation due to the ring structure of its side chain. As the triple‐helix already has a structure with the minimum energy due to the closely wound hydrogen‐bonded chains and the regular pattern of the amino acid residues, changes to its conformation to provide an entropic contribution to its free energy are not required. Thus, collagen molecules maintain a stable triple‐helix structure, unlike globular proteins that can undergo conformational changes.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Materials for Biomedical Engineering»
Представляем Вашему вниманию похожие книги на «Materials for Biomedical Engineering» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Materials for Biomedical Engineering» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












