The building blocks of simple proteins are approximately 20 naturally occurring α‐amino acids ( Figure 2.17; Table 2.4). These amino acids have a characteristic composition that includes an amine (NH 2) group at one end of the molecule and a carboxyl (C=O)OH group at the other end. The molecules also have a side‐group, denoted generally as R, attached to the carbon atom between the NH 2and (C=O)OH groups, which can be nonpolar, polar, positively charged or negatively charged. The simplest amino acid, glycine has R = H, the hydrogen atom. Proteins form by a condensation reaction involving the carboxyl group of one amino acid with the amine group of another amino acid, resulting in an amide bond between the two amino acids ( Figure 2.18a). Conventionally, in drawing such structures, the NH 2group of the amino acid or the N terminus of the protein is on the left whereas the (C=O)OH group of the amino acid or C terminus of the protein is on the right.
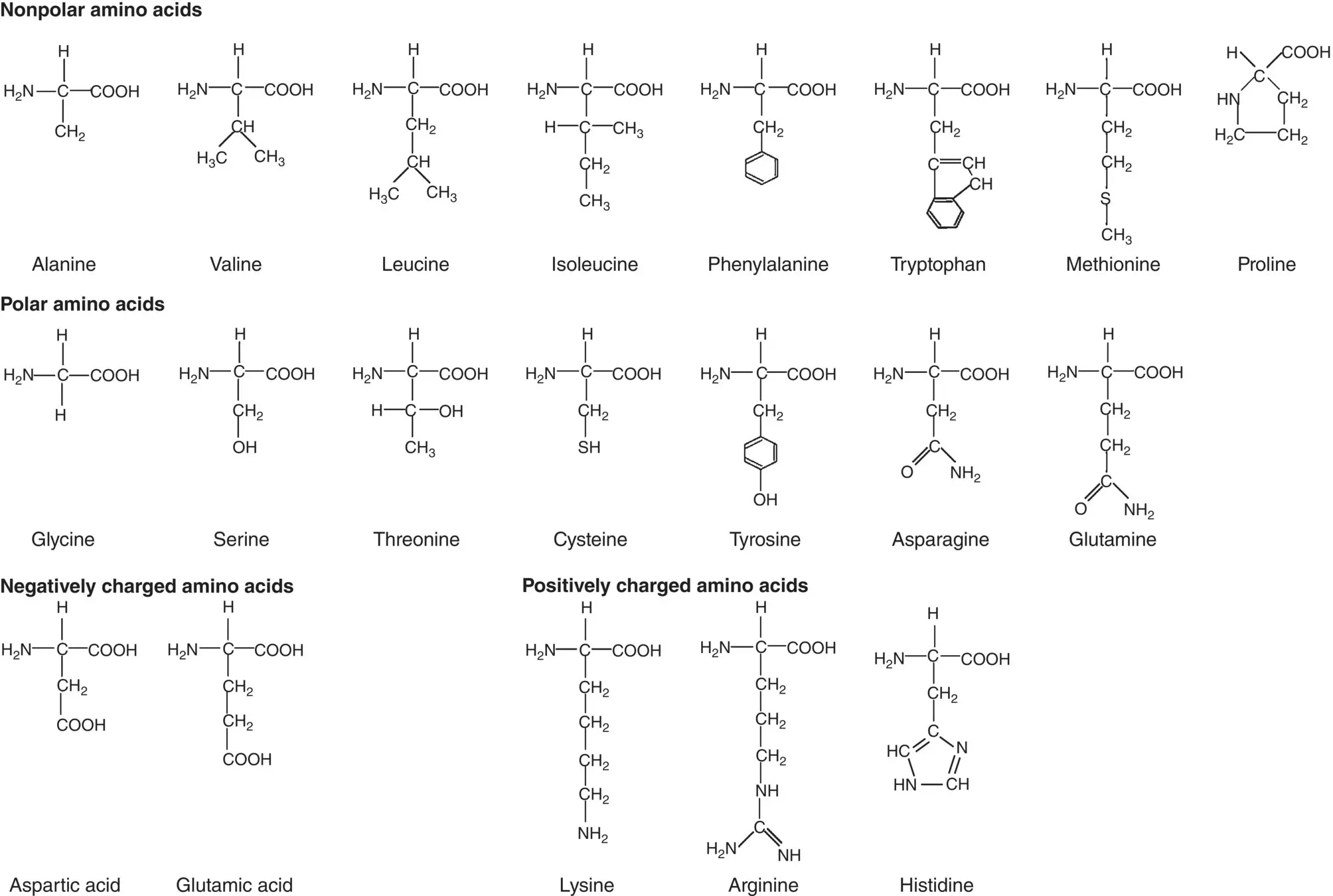
Figure 2.17 Side groups in 20 naturally occurring α‐amino acids which can be nonpolar, polar, negatively charged, or positively charged.
Table 2.4 The α‐amino acids of proteins.
| Amino acid |
Three‐letter symbol |
One‐letter symbol |
p K avalue of side chain |
| Alanine |
Ala |
A |
|
| Valine |
Val |
V |
|
| Leucine |
Leu |
L |
|
| Isoleucine |
Ile |
I |
|
| Phenylalanine |
Phe |
F |
|
| Tryptophan |
Trp |
W |
|
| Methionine |
Met |
M |
|
| Proline |
Pro |
P |
|
| Glycine |
Gly |
G |
|
| Serine |
Ser |
S |
|
| Threonine |
Thr |
T |
|
| Cysteine |
Cys |
C |
|
| Tyrosine |
Tyr |
Y |
|
| Asparagine |
Asn |
N |
|
| Glutamine |
Gln |
Q |
|
| Aspartic acid |
Asp |
D |
3.9 |
| Glutamic acid |
Glu |
E |
4.3 |
| Lysine |
Lys |
K |
10.5 |
| Arginine |
Arg |
R |
12.5 |
| Histidine |
His |
H |
6.0 |
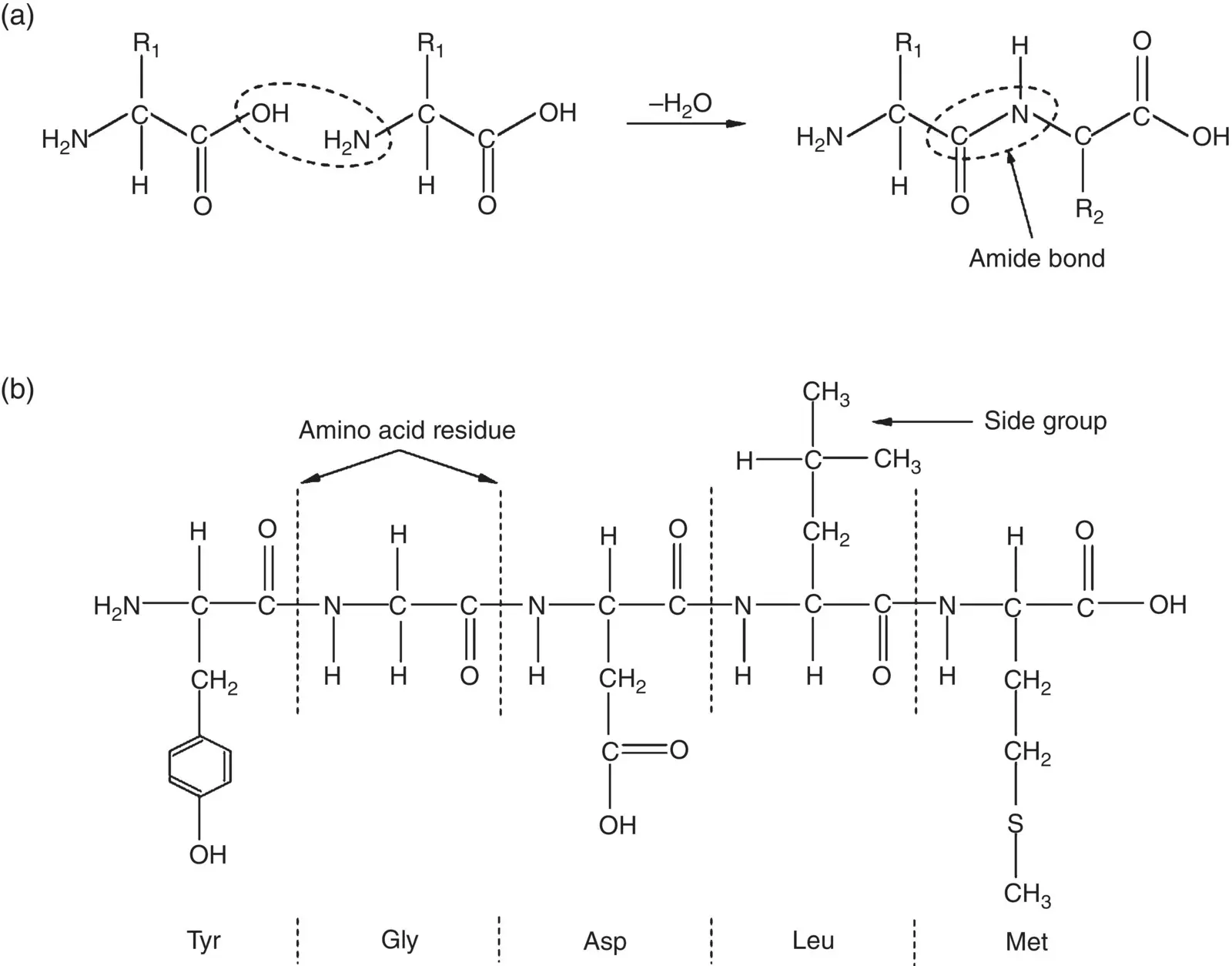
Figure 2.18 Illustration of (a) condensation reaction between two amino acids resulting in the formation of an amide (peptide) bond, and (b) peptide composed of five amino acid residues, showing the atomic bonds in the chain backbone and side groups. The three‐letter symbol of each amino acid is given below its residue.
A key feature of protein molecules is the presence of amide bonds in the chain backbone. These bonds are present in other macromolecules as well, such as in the synthetic polymer nylon 6.6 and other nylons, for example ( Figure 2.16b). However, when it involves two natural amino acids, it is sometimes called a peptide bond. While there is no clear agreement on terminology, a protein molecule is often taken as composed of over 50–60 amino acid groups often called amino acid residues. Smaller molecules composed of less than 10–20 residues are called peptides whereas molecules composed of more than 10–20 residues are often called polypeptides. Consequently, a protein molecule is often called a polypeptide also.
Proteins are large molecules, composed of ~50–2000 amino acid residues, but the peptide illustrated in Figure 2.18b, composed of five residues, shows the key features of the primary bonds in the chain backbone and examples of side groups attached to the chain backbone. The sequence of the amino acid residues in the chain backbone is called the primary structure of the protein. This primary structure dictates the higher‐order structure of proteins.
2.6.2 Secondary Structure
The term secondary structure describes the geometry of the long‐chain backbone of the individual protein macromolecule. This geometry can consist of randomly coiled chain along with regions composed of a regular repeating pattern. It is strongly influenced by two key factors
The stereochemistry of the amide bond
Hydrogen bonding between the oxygen atom of the carbonyl (C=O) group and the hydrogen atom of the amino (N–H) group in the chain backbone.
Stereochemistry of the Amide Bond
A key feature of the amide bond is that the carbonyl (C=O) double bond resonates between the C–O and C–N positions, which gives the C–N bond partial double bond character ( Figure 2.19a). The N atom has trigonal planar geometry, in a manner similar to carbon atoms in the ethylene molecule due to sp 2hybridized electron orbitals ( Section 2.4.2). Because rotation about a double bond is restricted, the amide bond has a planar geometry ( Figure 2.19b). This has important consequences for the secondary and tertiary structure of the protein molecule.
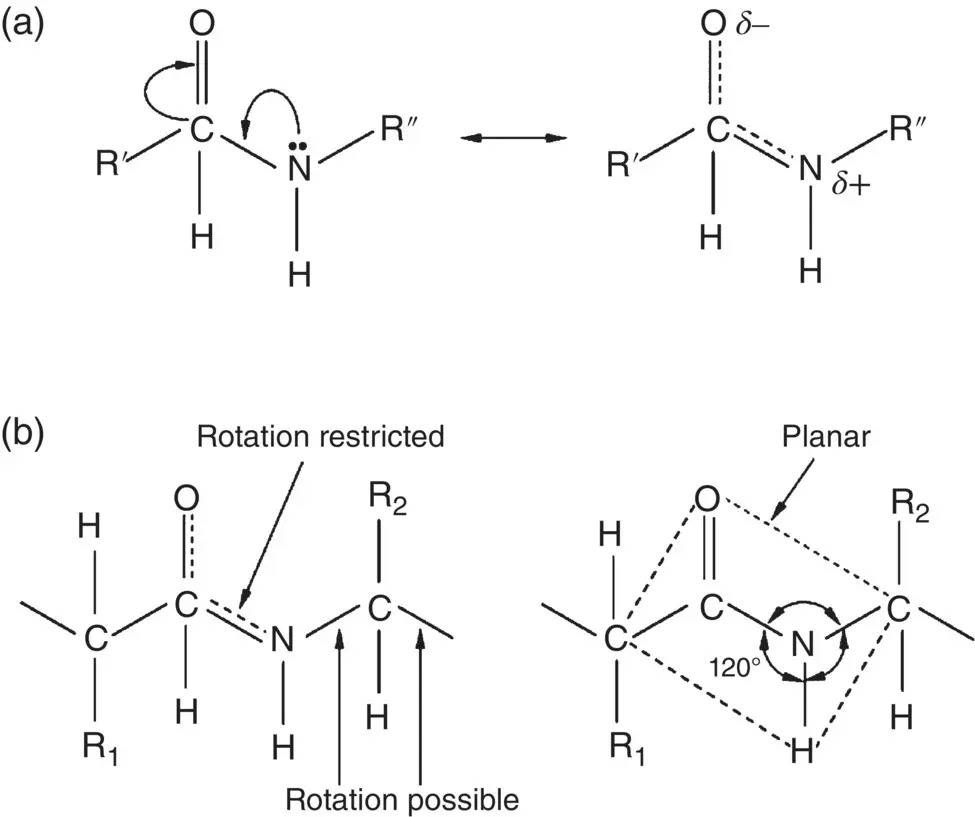
Figure 2.19 Schematic illustration of the stereochemistry of the amide bond. (a) Resonance of the carbonyl (C=O) double bond, resulting in partial double bond character of the amine (N–H) bond. (b) Planar geometry of amide bond.
Due to electronegativity differences between the atoms ( Section 2.4), the carbonyl C=O and amino N–H bonds are polarized. Thus, the O and H atoms in these bonds have a partial negative ( δ −) and positive charge ( δ +), respectively. As the O atom also has lone pair electrons, this leads to hydrogen bonding between the O and H atoms of different amide groups that can occur within a chain (intrachain bonding) and between neighboring chains (interchain bonding). Hydrogen bonding is an important feature of both the secondary and higher order structures of proteins but the way it occurs within the chain controls the secondary structure.
While the overall conformation of a protein is complex and unique, a feature of the chains themselves is that they often contain regions with two regular repeating patterns called the α‐helix and the β‐sheet. These two structures result from hydrogen bonding between the O atoms of the C=O group and the H atoms of the N–H group of the amide bond in the chain backbone, without involving the side chains.
An α‐helix is generated when a single protein chain twists around itself in the form of a helical geometry ( Figure 2.20). Hydrogen bonds between every fourth peptide bond stabilizes the structure, giving a regular helix with a complete turn every 3.6 amino acid residues. To reduce steric hindrance to this type of intrachain bonding, the side groups attached to the chain backbone point outward from the chains. Other helices occur but they are less common.
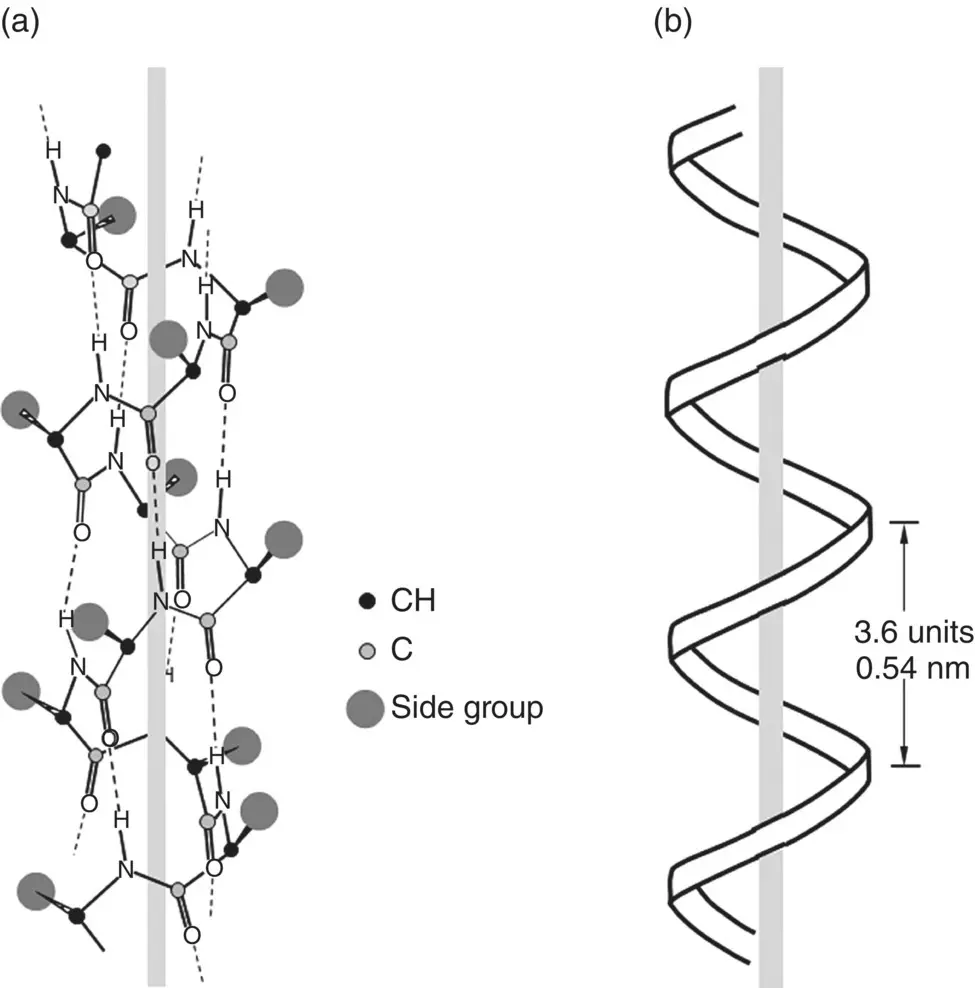
Figure 2.20 Illustration of (a) α‐helix structure generated by intrachain hydrogen bonding in polypeptides, and (b) geometrical parameters of α‐helix. The side groups point outward from the chain.
Читать дальше
















