Mohamed N. Rahaman - Materials for Biomedical Engineering
Здесь есть возможность читать онлайн «Mohamed N. Rahaman - Materials for Biomedical Engineering» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Materials for Biomedical Engineering
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Materials for Biomedical Engineering: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Materials for Biomedical Engineering»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
A comprehensive yet accessible introductory textbook designed for one-semester courses in biomaterials Materials for Biomedical Engineering: Fundamentals and Applications
Materials for Biomedical Engineering: Fundamentals and Applications
Materials for Biomedical Engineering — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Materials for Biomedical Engineering», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
2.4.2 Covalent Bonding
Covalent bonding involves sharing of electrons between two atoms but the shared electrons are localized between the two atoms and do not move freely. Depending on the number of valence electrons, atoms can each share one or more electrons, giving single, double or triple bonds. Common examples include hydrogen (H 2) and chlorine (Cl 2) molecules that share one electron each (single bond), oxygen (O 2) sharing two electrons each, and nitrogen (N 2) molecules sharing three electrons each.
Hybrid Orbitals
Some atoms such as those with electrons in their s, p, and d subshells, for example, can also form hybrid orbitals, each orbital being equivalent and having the same energy. As carbon, nitrogen and oxygen atoms are common constituents of many biomaterials, they are used here to illustrate the formation of hybrid orbitals. The 2s orbital and the three 2p orbitals in the carbon atom, for example, can combine to form four sp 3orbitals, each containing one electron ( Figure 2.6). Formation of these hybrid orbitals can lead to an overall reduction in energy and, thus, to a more stable compound when the atom with the hybridized orbitals forms a bond with other atoms. Nitrogen and oxygen atoms can also form sp 3hybrid orbitals but one orbital in nitrogen and two orbitals in oxygen are filled with two electrons. As they are not available for bonding with other atoms, the two electrons in each of the filled orbitals are referred to as lone‐pair electrons.
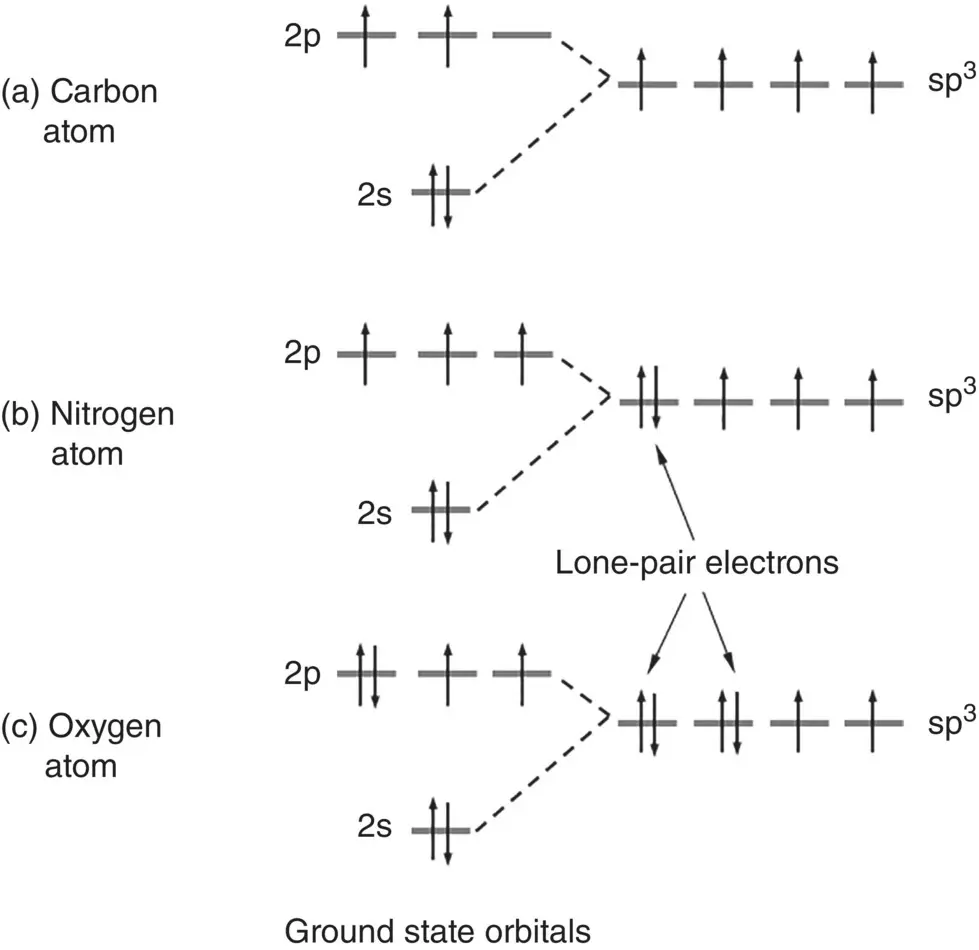
Figure 2.6 Schematic illustration of the formation of four sp 3orbitals in (a) the carbon atom, (b) the nitrogen atom, and (c) the oxygen atom. Each hybrid orbital in carbon can form a covalent bond by sharing an electron from another atom. In comparison, one orbital in nitrogen and two orbitals in oxygen are filled with two electrons, called lone‐pair electrons, which are not available for covalent bonding but available for hydrogen bonding.
Hybrid orbitals are formed at specific angles to one another and, as bonds are formed in the directions of greatest orbital overlap, these orbitals give directionality to the bond that, in turn, determine the arrangement of the atoms in three dimensions and how the atoms pack to form solids. Hybridization of s and p orbitals, for example, can occur in the following ways ( Figure 2.7):
One s orbital and one p orbital to form two sp orbits that are diametrically opposed to each other. The angle between the orbitals is 180°, giving a linear arrangement of the orbitals
One s orbital and two p orbitals to form three sp2 orbitals at 120° to one another. The orbitals form a triangular arrangement in a single plane and they are said to form a trigonal planar arrangement
One s orbital and three p orbitals to form four sp3 orbitals at 109.5° to one another. The orbitals point to the corners of a tetrahedron.
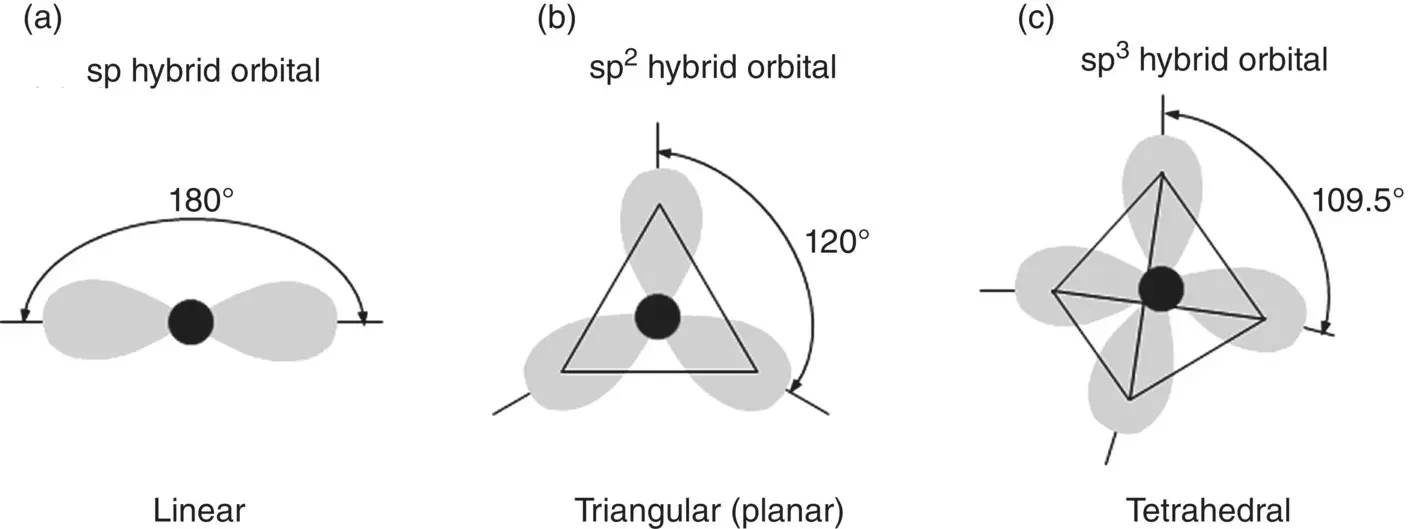
Figure 2.7 Schematic comparison of the directionality of sp, sp 2, and sp 3hybrid orbitals.
When a carbon atom bonds with four hydrogen atoms to form a methane molecule (CH 4), for example, the shared electrons point to the corners of a tetrahedron ( Figure 2.8). On the other hand, when oxygen or nitrogen atoms with sp 3hybridized orbitals bond with other atoms such as hydrogen atoms to form water (H 2O) or ammonia (NH 3) molecules, respectively, the angle between the OH bonds in H 2O or between the NH bonds in NH 3differs slightly from 109.5° due to the presence of the lone‐pair electron orbitals. The bonds and lone‐pair electron orbitals form a slightly distorted tetrahedral arrangement, with the angle between the OH bonds in H 2O and between the NH bonds in NH 3having a value of 104.5° and 107°, respectively.
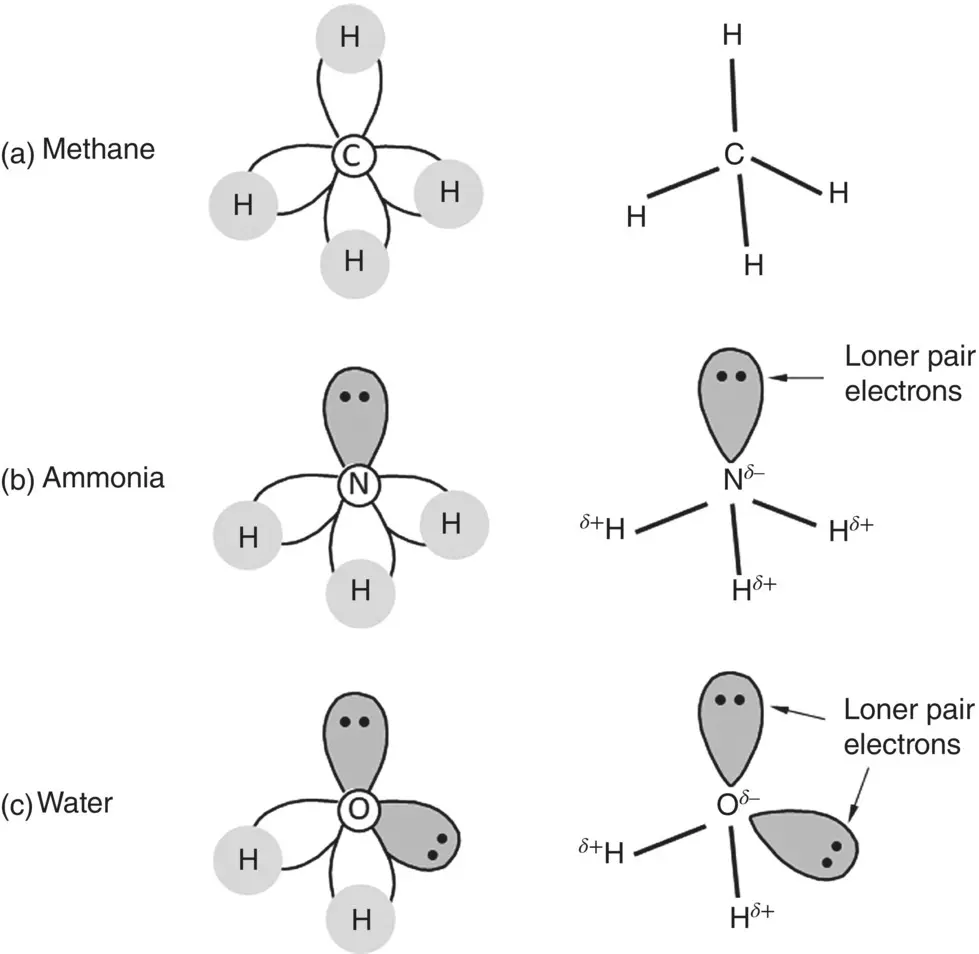
Figure 2.8 Schematic illustration of covalent bonding in methane, ammonia, and water molecules. The polarity of the bonds and lone pair electrons in ammonia and water are also shown.
The hybridized electron orbitals in atoms such as carbon, nitrogen, and oxygen can also lead to the formation of double and triple bonds when these atoms bond to the same or other atoms. The oxygen molecule (O 2), for example, has a double bond between the atoms while the nitrogen molecule (N 2) has a triple bond between the atoms. To visualize the formation of single, double, and triple bonds between atoms, we can compare the structures of the hydrocarbon molecules ethane (C 2H 6), ethylene (C 2H 4), and acetylene (C 2H 2) ( Figure 2.9). The formation of the ethane molecule, for example, can be viewed in terms of bonding among two carbon atoms, each having four sp 3hybrid orbitals, and hydrogen atoms. Each bond in the molecule shares two electrons and points directly at each other along the internuclear axis. This type of bond is called a sigma (σ) bond and it is the most common type of covalent bond.
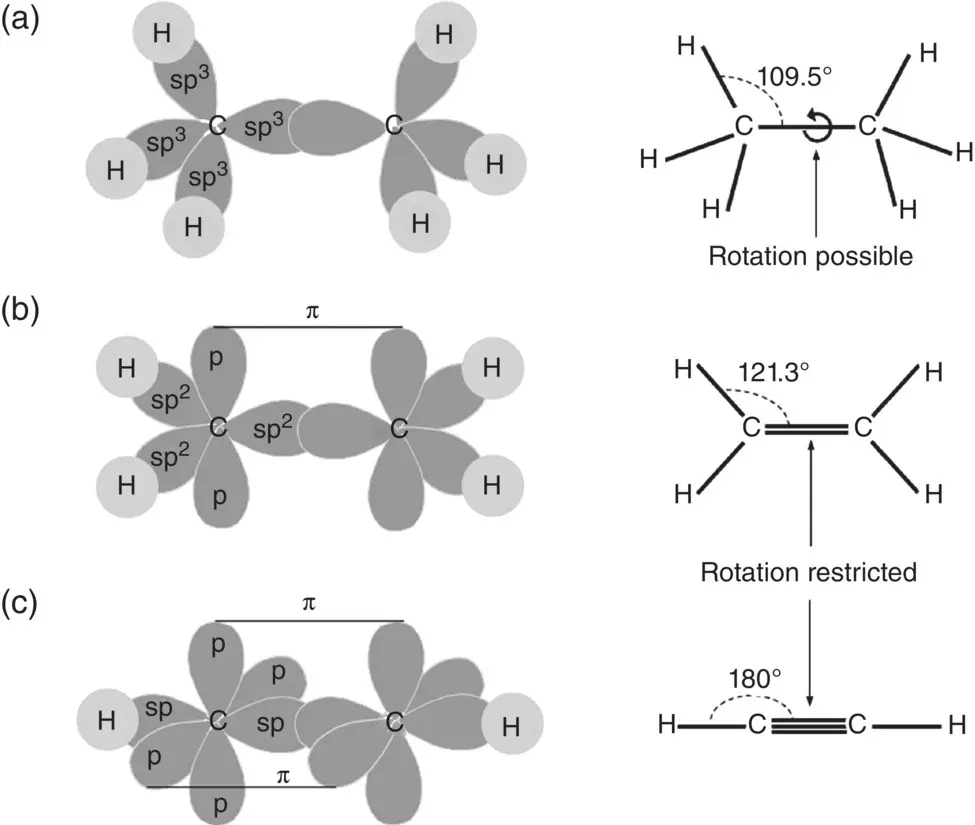
Figure 2.9 Illustration of the formation and geometry of single, double, and triple bonds in (a) ethane, (b) ethylene, and (c) acetylene.
In the ethylene molecule, each carbon atom has three sp 2hybrid orbitals and one unhybridized p orbital. Each sp 2orbital forms a σ bond with another carbon atom and with a hydrogen atom. In contrast, the two p orbitals, one from each carbon atom, can share their electrons in a side‐by‐side manner, perpendicular to the internuclear axis, to complete the valence shell of the carbon atom. This type of bond is called a pi (π) bond. The combination of the σ and π bonds results in a double bond between the carbon atoms. In the acetylene molecule, we have one σ bond between the two carbon atoms, due to sp hybrid orbitals in each carbon atom, plus two π bonds from the two unhybridized p orbitals, making a triple bond between the carbon atoms.
In the case of the hydrocarbons, such as ethane, ethylene, and acetylene, for example, a triple bond between two carbon atoms has a higher bonding energy and a shorter interatomic distance than a double that, in turn, has a higher bonding energy and shorter interatomic distance than a single bond. Another important distinction between the single, double, and triple bonds is the freedom of one carbon atom to rotate about the internuclear axis with the other carbon atom. As the energy barrier for rotation is low, molecules can rotate freely around a single carbon–carbon bond. On the other hand, rotational motion is highly restricted around a double or a triple carbon–carbon bond. The ethylene molecule has a planar shape whereas the acetylene molecule is linear. Similar trends hold for bonds between other pairs of atoms, such as between carbon and nitrogen atoms. Overall, then, the presence of double and triple bonds has important consequences for the structure and, thus, the properties and function of covalent‐bonded molecules.
Covalent Bonding in Ceramics
Covalent‐bonded ceramics are characterized by intrinsic properties similar in nature to those described for ionic‐bonded ceramics ( Section 2.6) but as the covalent bond can be stronger than the ionic bond, many covalent‐bonded ceramics have better mechanical properties, such as strength, elastic modulus and hardness, and a higher melting point.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Materials for Biomedical Engineering»
Представляем Вашему вниманию похожие книги на «Materials for Biomedical Engineering» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Materials for Biomedical Engineering» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












