Mohamed N. Rahaman - Materials for Biomedical Engineering
Здесь есть возможность читать онлайн «Mohamed N. Rahaman - Materials for Biomedical Engineering» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Materials for Biomedical Engineering
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Materials for Biomedical Engineering: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Materials for Biomedical Engineering»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
A comprehensive yet accessible introductory textbook designed for one-semester courses in biomaterials Materials for Biomedical Engineering: Fundamentals and Applications
Materials for Biomedical Engineering: Fundamentals and Applications
Materials for Biomedical Engineering — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Materials for Biomedical Engineering», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Empirical relations based on electronegativity values have been proposed to predict or compare the type of bond but they provide only an approximation and involve some degree of arbitrariness. One simple relation, based on the Pauling electronegativity scale, considers the difference in electronegativity, ΔEN, as follows:
ΔEN > 2.0: ionic bond
ΔEN < 0.4: covalent bond
0.4 < ΔEN < 2.0: combination of ionic and covalent character
While these ΔEN values are useful for predicting whether a bond is predominantly ionic or covalent, there is no sharp distinction between an ionic bond and a bond that has a combination of ionic and covalent character, and between a covalent bond and a bond that has a combination of ionic and covalent character. Empirical equations have been developed to predict the percentage of ionic character I of a bond between two atoms with a difference in electronegativity ΔEN. One such equation is
(2.5) 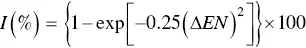
However, the exact nature of a bond that has a certain percentage of ionic and covalent character is unclear.
Polarity of Covalent Bond
The formation of an ionic bond involves the transfer of one electron or more from one atom to the other, creating ions with discrete positive and negative charges ( Figure 2.1a). On the other hand, the covalent bond involves sharing of electrons between two atoms ( Figure 2.1b). When the atoms forming the covalent bond are the same, such as two hydrogen atoms or two chlorine atoms to form a hydrogen molecule (H 2) or a chlorine molecule (Cl 2), the bonding electrons are shared equally between the two atoms ( Figure 2.5a). However, when the two atoms are different, such as a hydrogen atom and a chlorine atom forming a hydrogen chloride molecule (HCl), the bonding electrons in the covalent bond are not shared equally between the atoms. The chlorine atom has a greater capacity to attract the shared electrons to itself, so due to its higher electronegativity, the shared electrons are attracted more strongly to the chlorine atom than the hydrogen atom ( Figure 2.5b). Because of this unequal sharing, the chlorine atom develops a slightly negative character whereas the hydrogen atom develops a slightly positive character. The covalent bond is said to be polar, to consist of a dipole or to have a dipole moment. When appropriate to emphasize the polar nature of the molecule, the more electronegative atom in the bond is shown with a partial negative charge δ −symbol while the less electronegative atom is shown with a δ + symbol ( Figure 2.5).
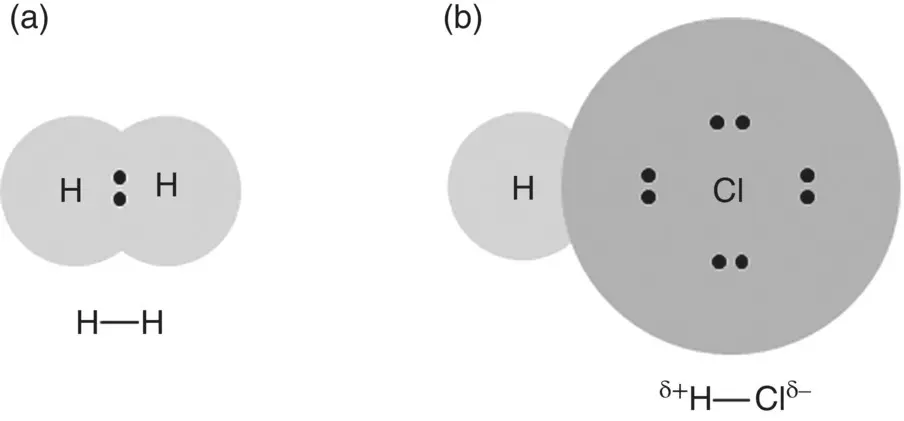
Figure 2.5 Illustration of (a) nonpolar bond in hydrogen molecule (H 2) and (b) polar bond in hydrogen chloride molecule (HCl).
2.4.1 Ionic Bonding
The electrons in each ion occupy orbitals of complicated shape but, at an approximate level, we can assume that the ions have a spherical shape. Thus, in ionic bonding, one ion can be attracted to the other in any direction and, for the same interatomic distance, the force of attraction does not depend on the direction. We say that that ionic bond lacks directionality or is nondirectional. In packing to form a three‐dimensional structure, one type of ion will surround itself with as many ions of the other type as far as possible to maximize the attraction between cations and anions and to minimize the repulsion between ions of the same charge ( Chapter 3). In this way, the attractive potential energy of the system is maximized. Another feature of ionic bonding is that in the creation of ions, an electron transferred from one atom to the other atom remains localized at this second atom and cannot move freely. These features of the ionic bond provide a basis for understanding the characteristic properties of ionic solids (Section 1.4):
High strength, high elastic modulus, high melting point, and chemically unreactive due to the strong interatomic bonding
Low electrical and thermal conductivity due to the localized electrons
Brittleness: Permanent deformation of an ionic solid due to a large enough mechanical force or stress requires motion of ions from their equilibrium sites to adjacent equilibrium sites. For this to occur, ions in one plane of the solid must move over or near to ions of the same sign in the adjacent plane. This leads to a strong repulsion and to catastrophic fracture of the solid into two or more pieces
High hardness: As hardness is related to the resistance of the material to be permanently deformed, ionic solids typically show high hardness
Ceramics are held together by ionic or covalent bonds, or by bonds with a mixture of ionic and covalent character. Although they are not useful as biomaterials, sodium chloride (NaCl), and magnesium oxide (MgO) are common examples of ceramics that show ionic bonding. Ceramic biomaterials that show predominantly ionic bonding include aluminum oxide (Al 2O 3) used as bearings in some hip implants, and the calcium phosphates hydroxyapatite, Ca 10(PO 4) 6(OH) 2, and tricalcium phosphate, Ca 3(PO 4) 2, used to repair bone defects (Table 1.1).
Example 2.1
Sodium chloride (NaCl) and magnesium oxide (MgO) are both ionic solids and they have the same crystal structure. However, MgO has a melting temperature (2800 °C) which is far higher than that of NaCl (801 °C). Explain why.
Solution:
Two factors are mainly responsible: (i) the charge of the ions and (ii) the size of the ions, taken as the ionic radius, which determines the equilibrium interatomic spacing.
1 Charge of the ions: The sodium atom (atomic number = 11; ground state configuration = 1s22s22p63s1) loses one electron to become a sodium ion (Na+) while the chlorine atom (atomic number = 17; ground state configuration = 1s22s22p63s23p5) gains one electron to become a chlorine ion (Cl−). In comparison, the magnesium atom (atomic number = 12; ground state configuration = 1s22s22p63s2) loses two electrons to become a magnesium ion (Mg2+) while the oxygen atom (atomic number = 8; ground state configuration = 1s22s22p4) gains two electrons to become an oxygen ion (O2−). The attractive potential energy ( Eq. (2.3)) between the ions depends on the product of the ionic charges. For NaCl, this is (1 × 1) whereas for MgO, this is (2 × 2). Thus, for a given distance of separation, the attractive potential energy for the MgO bond is four times that for the NaCl bond.
2 Size of the ions: The electronic configuration of the Na+ and Mg2+ ions are the same (1s22s22p6). On the other hand, the Mg2+ ion has a higher nuclear charge than the Na+ ion and, thus, it has a smaller ionic radius due to the greater attraction between the nucleus and the electrons remaining in the ion. The O2− ion has an electronic configuration (1s22s22p6) composed of one less shell than the Cl− ion (1s22s22p63s23p6). Although the oxygen atom gains two electrons to form an O2− ion whereas the chlorine atom gains one electron to form a Cl− ion, it might be expected that the O2− ion will have a somewhat smaller radius due to it having one less electron shell. Thus, the equilibrium interatomic separation of the molecule, taken approximately as the sum of the ionic radii, is smaller in MgO than in NaCl.
We see from Eq. (2.3)that the potential energy of attraction is proportional to the product of the charges and inversely proportional to the interatomic distance. Thus, based on the ionic charges and interatomic separation, the attractive potential energy at a separation approximating the equilibrium separation distance for MgO is at least four times higher than for NaCl. Although we have not taken into account the repulsive potential energy, Figure 2.2indicates that it is far smaller than the attractive potential energy. Overall, it will take a much larger amount of energy to disrupt the MgO bond and, thus, the melting point of MgO is much higher than that of NaCl.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Materials for Biomedical Engineering»
Представляем Вашему вниманию похожие книги на «Materials for Biomedical Engineering» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Materials for Biomedical Engineering» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












