Source: Adapted from Ref. [9] with permission from John Wiley and Sons.
This mechanism can be concluded as the restriction of access to the dark state (RADS), which further elucidates the connotation of the RIM mechanism. The previous investigation mainly focuses on the NAC between the first excited state and the ground state. As an extreme scenario of NAC, the CI can cause ultrafast deactivation. In fact, the PES of multiple excited states can be coupled by molecular motions and arranged in a complicated manner, especially for the heteroatom‐containing molecular systems. The accessible dark states like the CT state, ( n , π *) state, and the triplet state will cause the fluorescence quenching. Once the molecular motions that lead to the dark states undergo the intramolecular or environmental constraints, the fluorescence can be restored. The multistate model has been proved effective to evaluate the excited‐state deactivation of the heteroatom‐containing luminogens.
1.5 Suppression of Kasha’s Rule
For classical luminophores, the internal conversions from higher excited states to the lowest excited states are much faster than the luminescence processes due to the rigid structures and large conjugation even in the gas phase or dilute solution, so the light emission always comes from the lowest excited state with a given spin multiplicity [20].
However, most AIEgens possess highly flexible structures with substantial low‐frequency molecular motions that are sensitive to the environmental constraints. The vigorous molecular motions will contribute to the coupling and mixing of multiple excited states. Once the relevant molecular motions are restricted in the aggregate or solid state, it is possible that the excitons can be stabilized in higher excited states and generate anti‐Kasha light emissions.
Qian et al., first, proposed the suppression of Kasha’s rule (SOKR) as the mechanism for the AIE behaviors of molecular rotors based on the boron‐difluorohydrazone (BODIHY) [11a]. The luminescence properties related to higher excited states of the BODIHY derivatives in the solution have been studied through the spectroscopy by changing the viscosity of the solution and varying the excitation wavelength. These derivatives show viscosity‐dependent emission enhancement but nearly no response to the solution polarity due to weak partial charge transfer. According to the calculation at the TDA‐PBE level, the first excited state is designated as a dark state. Instead, the S 3state is a bright state, which is more populated due to the relatively large energy gap between the S 3and lower excited states. It is demonstrated that once the viscosity increases, the rotation of the phenyl rings can be hindered and make the excitons stabilized in the higher excited state to generate enhanced anti‐Kasha emission. However, these results still remain controversial. Zhou et al. have recently employed more DFT functions to recheck the excited‐state properties of the BODIHY derivatives and have challenged the SOKR mechanism [11b]. They have found that the TDA‐PBE method used in Ref. [11a] may not describe the correct order of the excited states, and the energy gaps between S 3and S 2states obtained from this method are small enough to generate efficient internal conversion from S 3and S 2states. Hence, the emission of BODIHY derivatives in higher viscosity may not be induced by the SOKR. Instead, they proposed that the restriction of access to the CI caused by the flip‐flop motion is responsible for the AIE behaviors of BODIHY derivatives.
Guo et al. has recently reported another luminogen named DMF‐BP‐PXZ with highly efficient light emission from the S 2state ( Figure 1.9a, b) [11c]. According to the calculation, the S 1state is a transition‐forbidden dark state, and the internal conversion dominates the decay process in the solution due to the severe molecular motions, so the rapid internal conversion from S 2to S 0state, through the intermediate S 1state, quenches the light emission. However, its k ICfrom S 2to S 0can be suppressed by four orders of magnitude, and the fluorescence radiation rate can be enhanced in the solid state, which leads to the efficient light emission from S 2state to the ground state. In this regard, further experimental evidence for the stable population of higher excited states is still highly desired to solidify the claim on the anti‐Kasha emission, but the mechanism demonstrated earlier also belongs to the category of RIM, and it validates the wide reliability of the RIM mechanism.
Exciton population on the higher excited states can also occur in the triplet states. Taking the ClBDBT as an example ( Figure 1.9c, d), it exhibits white‐light emission under UV light and persistent yellow afterglow in the room temperature [11d]. According to the calculated energy levels, T 1and T 2states are all lower than the S 1state in energy, which makes both T 1and T 2accessible for the exciton population coming from the S 1state. Furthermore, the T 2state mainly contains the ( n , π *) transition character, which leads to a larger spin–orbit coupling (SOC) between T 2and the S 0and a higher radiative decay rate, whereas the T 1state contains more ( π , π *) transition character. At room temperature, the small energy gap between T 2and T 1can promote the thermal population from T 1to T 2. According to Boltzmann distribution, T 2has a smaller population than T 1, but the faster radiative decay from T 2results in a balanced emission intensity from both T 2and T 1states. Thus, the combined anti‐Kasha blue light from T 2and the yellow light from T 1generate the efficient white‐light emission at room temperature.
In fact, it is also the RIM process in the crystal state that stabilizes the specific electronic structures of T 2and T 1and restricts the nonradiative decay from triplet states to the ground state, and then the balanced dual emission can be restored.
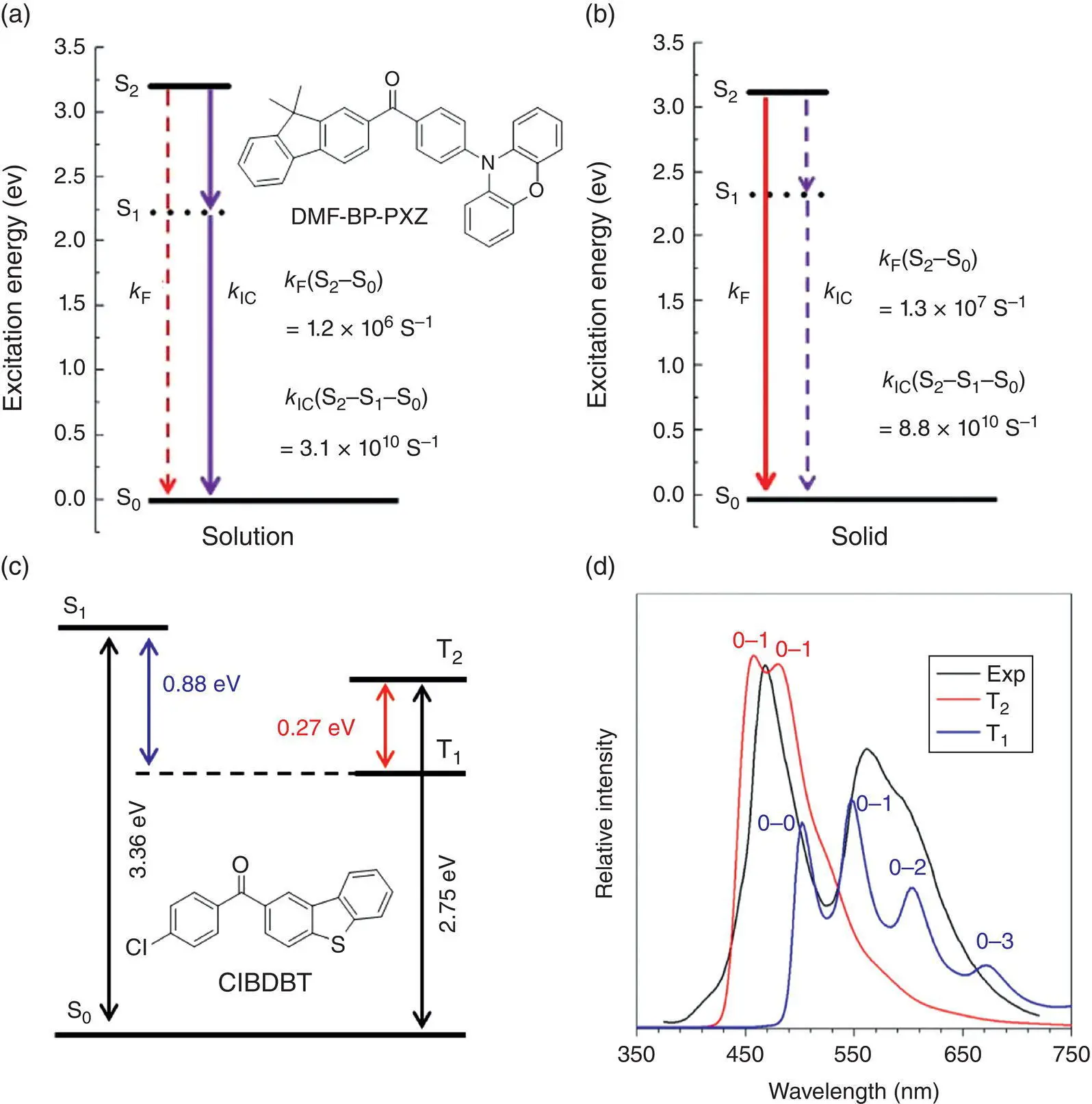
Figure 1.9 Molecular structure, calculated energy levels, fluorescence, and internal conversion rate constants of DMF‐BP‐PXZ in the (a) solution and (b) solid state.
Source: Adapted from Ref. [11c] with permission from John Wiley and Sons.
(c) Molecular structure, calculated energy levels, and (d) emission spectra of ClBDBT in the solid state.
Source: Adapted from Ref. [11d] with permission from Springer Nature.
1.6 Through Space Conjugation
Most of the classical AIEgens are constructed by chromophores with through‐bond conjugation (TBC), and their emission can be enhanced through restricting the nonradiative decay driven by molecular motions, whereas the light emission can also be boosted by promoting the radiative rates through the through‐space conjugation (TSC) [13]. The TSC plays a key role in radiative decay processes of molecular systems with clusterization‐triggered emission (CTE) property [21]. Moreover, a certain degree of molecular motions in the solid state will facilitate the intra‐ or intermolecular excited‐state TSC for the nonconjugated molecules and stabilize the radiative channels and, thus, promote the emission intensity [13].
1.6.1 Clusterization‐Triggered Emission
Traditional luminogens, generally, consist of aromatic groups or other conjugated building blocks and take the two‐dimensional conjugated structure connected by chemical bonds. In such molecular systems with TBC, the EVC between emissive states and the ground state, the access to CIs, or the dark states driven by molecular motions are usually the detrimental causes for emission quenching. However, researchers have discovered and developed a nonconventional type of luminogens without any long‐range conjugated structures but containing only isolated units such as heteroatoms with lone‐pair electrons, unsaturated C=C, C=O, and C≡N groups as presented in Figure 1.10. Although this kind of luminogens lacks luminescent centers intuitively, and they are almost nonemissive in the dilute solution, they can emit visible light efficiently in the aggregate or solid state, showing the typical AIE behaviors. Because of the lack of largely conjugated luminescent centers and much higher motion ability than the traditional molecules with long‐range conjugation, such nonconjugated molecules are nonemissive in the isolated state. However, it can be noticed that these heteroatom‐containing groups usually exist as amide, imide, ketone, anhydride, or ester subunits connected by the saturated bonds in the macromolecule backbones, and they are usually rich in electrons. Hence, once the aggregation or cross‐linking occurs, electron‐rich moieties can cluster together and form a relatively stable long‐range through‐space electron ocean and energy bands by the electron overlapping for the electronic transition, which is similar to the energy band structures in the inorganic semiconductors. Upon excitation, electrons can jump into the excited state based on the stabilized through‐space energy bands and light up the whole cluster system.
Читать дальше













