Theoretically, the large EVC of most AIEgens is the main cause for their low emission efficiency in the isolated state, which originates from the nature of the conformational flexibility and high freedom of molecular motions of the AIEgens. Tang and coworkers have studied the substituent effect on the AIE behaviors of a series of stilbene derivatives, as presented in Figure 1.6, and verified the influence from freedom of molecular motions on the excited‐state properties [17b]. Three methyl groups can be attached to 2,4,5‐positions or 2,4,6‐positions of each phenyl rings of the stilbene, which leads to opposite photophysical behaviors. The 2,4,5‐TMe‐DPE shows aggregation‐caused quenching effect, whereas the 2,4,6‐TMe‐DPE shows the typical AIE behavior. The first question that arises is which factors make the 2,4,5‐substituted derivative highly emissive with Φ Fof 13.4% but the 2,4,6‐substituted counterpart almost nonemissive with Φ Fas 0.6% in the dilute solution. The quantum simulations in the ground state show that 2,4,5‐TMe‐DPE owns a more planar conjugation plane, while 2,4,6‐TMe‐DPE is more twisted. The calculated rotation barrier shows that 2,4,6‐TMe‐DPE undergoes lower barrier to rotate along the coordinate of twisting, but it suffers higher barrier to move along the coordinate of planarization, which reveals that methyl groups in 4,6‐positions can produce strong steric hindrance to prevent the coplanarization between phenyl rings and the double bond, but such steric hindrance can cause preliminary twisting of phenyl rings and reduce the conjugation degree, which leads to the easier torsional motions of the phenyl rings in 2,4,6‐TMe‐DPE. From the ultrafast spectroscopy measurement, the lifetime of excited‐state species of 2,4,6‐TMe‐DPE is only 2.1 ps, much shorter than that of 2,4,5‐TMe‐DPE (263 ps), which indicates that the 2,4,6‐TMe‐DPE is less conjugated and possesses higher motion ability, so it can undergo much faster excited‐state decay process.

Figure 1.5 (a) Chemical structures and the overlap of the S 1,minconformation and the S 0,minconformation in the gas and the solid state of HPS. (b) Diagonal elements R kkof the nonadiabatic electronic coupling matrix versus normal mode index in the gas and the solid state. (c) Reorganization energy versus normal mode wave numbers in the gas state and (d) the solid state.
Source: Adapted from Ref. [7a] with permission from American Chemical Society.
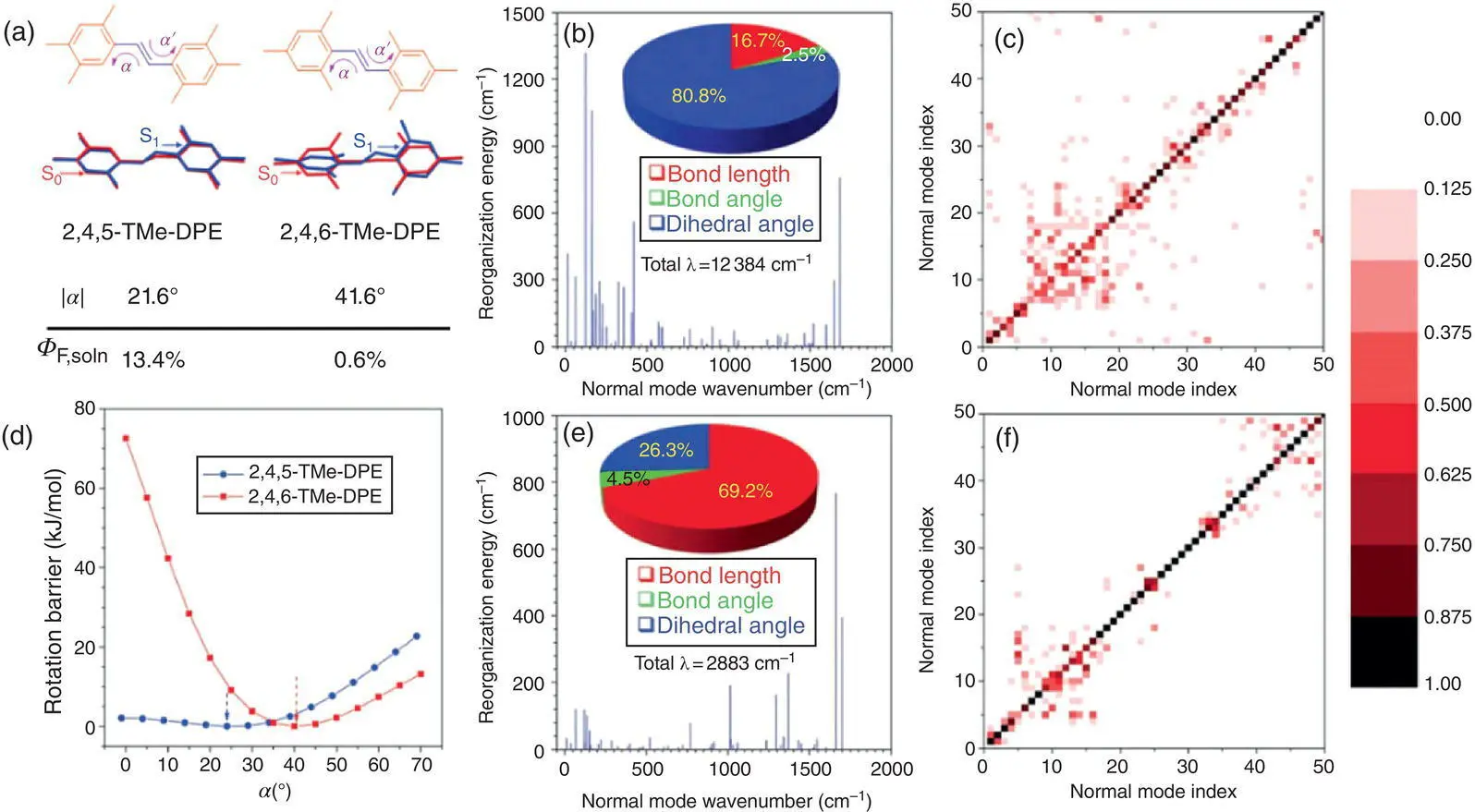
Figure 1.6 (a) Chemical structures, the overlaps of the S 1,minconformation and the S 0,minconformation in the gas phase, and fluorescence quantum yield in the dilute solution of 2,4,5‐TMe‐DPE and 2,4,6‐TMe‐DPE. Reorganization energy versus normal mode wave numbers in (b) the gas state and (e) the solid state of 2,4,6‐TMe‐DPE. Duschinsky rotation matrix of normal modes with index of 1–50 in (c) the gas state and (f) the solid state of 2,4,6‐TMe‐DPE. (d) Rotation barriers calculated through rotating the dihedral angle α .
Source: Adapted from Ref. [17b] with permission from The Royal Society of Chemistry.
Further EVC analysis is also consistent with the abovementioned result. The low‐frequency molecular motions dominate in isolated 2,4,6‐TMe‐DPE, which are mainly associated with torsion of the phenyl rings and twisting of the double bond in the excited state. Projection of RE onto the internal coordinate shows that over 80% of the total RE comes from the change in dihedral angles, which further verifies the higher twisting mobility of 2,4,6‐TMe‐DPE. The calculation of the Duschinsky rotation matrix (DRM) shows that the mixing of low‐frequency molecular motion modes of 2,4,6‐TMe‐DPE is much stronger than that of 2,4,5‐TMe‐DPE. The large area of nondiagonal elements on DRM indicates that 2,4,6‐TMe‐DPE owns multiple coupled decay pathways, connecting the excited‐state state with the ground state, through molecular motions, that can further enhance the nonradiative decay rate. On the contrary, the highly emissive 2,4,5‐TMe‐DPE shows much less RE and less mixed motion modes. In the crystal state, the RE of 2,4,6‐TMe‐DPE decreases to a large extent and the light emission is brightened. The abovementioned analysis draws a clear mechanistic picture of the AIE effect that high freedom of the isolated AIE molecule generates dominant low‐frequency molecular motions, rendering the excited‐state energy mainly dissipated through the nonradiative pathways; meanwhile, the intensive low‐frequency molecular motions can cause mixing among the motion modes, making the multiple decay pathways coupled together so that the nonradiative decay is further facilitated. These two factors cause the weak emission of AIEgens in the dilute solution. The low‐frequency molecular motions can be drastically restricted in the solid state due to their higher sensitivity to the environmental constraints, so the EVC can decrease to a large extent, leading to the decrease in the nonradiative decay rate, and then the luminescence quantum efficiency can be significantly boosted.
With persistent effort, researchers have revealed the importance of molecular motions to the luminescence process and triggered the experimental and theoretical studies on the molecular motions, and then, finally, they have concluded RIM, or theoretically the restriction of EVC, as the general working principle for AIE effect. RIM works well for explaining AIE behaviors for most AIEgens and serves as an effective design principle for the development of AIE materials. Recently, theoreticians have further explored specific features of PES in the excited states of AIEgens, considering the crossing between the excited state and the ground state and the coupling among the excited states.
1.3 Restricted Access to Conical Intersection
Previous experimental studies of molecular motions and theoretical analysis of EVC have elucidated the decay pathways of AIEgens, considering the starting and final equilibrium points on the first excited state and the ground state, respectively. Recently, theoreticians have investigated the dynamic features for both the excited state and the ground state of typical AIEgens by scanning the PES along specific molecular motion coordinates or by employing excited‐state molecular dynamics method, which has presented a clear mechanism picture of the photoinduced dynamic evolution for AIEgens [18].
When we look into the NAC between the two equilibrium points on the excited state and the ground state, based on the displacement harmonic approximation, it is the intensive low‐frequency modes that contribute to the energy conversion from the electronic form to the vibrational form. However, the massive molecular motions of a series of AIEgens can lead the excited‐state structures to evolve beyond the assumption of harmonic approximation to form highly twisted structures. In such cases, the conical intersections (CIs) between the excited state and the ground state will become the main cause for the fast nonradiative decay or channels for photochemical reactions [18a].
Taking the dimethyl tetraphenylsilole (DMTPS) as the model compound [18b], the central silole ring can undergo the alternation of the C=C double bond after absorption of photons, which further promotes its motion ability in the excited state. The potential energy profiles of the DMTPS in the excited state optimized by CASPT2//CASSCF method ( Figure 1.7b) show that the vibrational relaxation leads to a minimum point in the S 1state with 3.1 eV, at which the central silole ring still keeps planar, whereas along with the twisting of the silole ring, the potential energy profile experiences an energy barrier at 3.54 eV and, finally, leads to a CI point at 3.0 eV between the S 1and the S 0state, at which the silole ring turns into a highly twisted conformation, similar to the structural reorganization of the cis‐butadiene. Since the energy barrier separating the CI and the Frank–Condon region is lower than the vertical excitation energy, the CI is energetically accessible. Once the excited DMTPS species reach the CI, the NAC becomes infinitely large and finally results in the ultrafast nonradiative decay from the S 1state to the S 0state to quench the emission in the solution. But in the crystal, the QM/MM calculation of the potential energy profile shows that the CI is located at 4.91 eV, which is much higher than the vertical excitation energy of 3.65 eV, making the CI energetically inaccessible. Hence, the nonradiative decay through CI can be effectively blocked to recover the strong light emission.
Читать дальше














