1 ...7 8 9 11 12 13 ...40 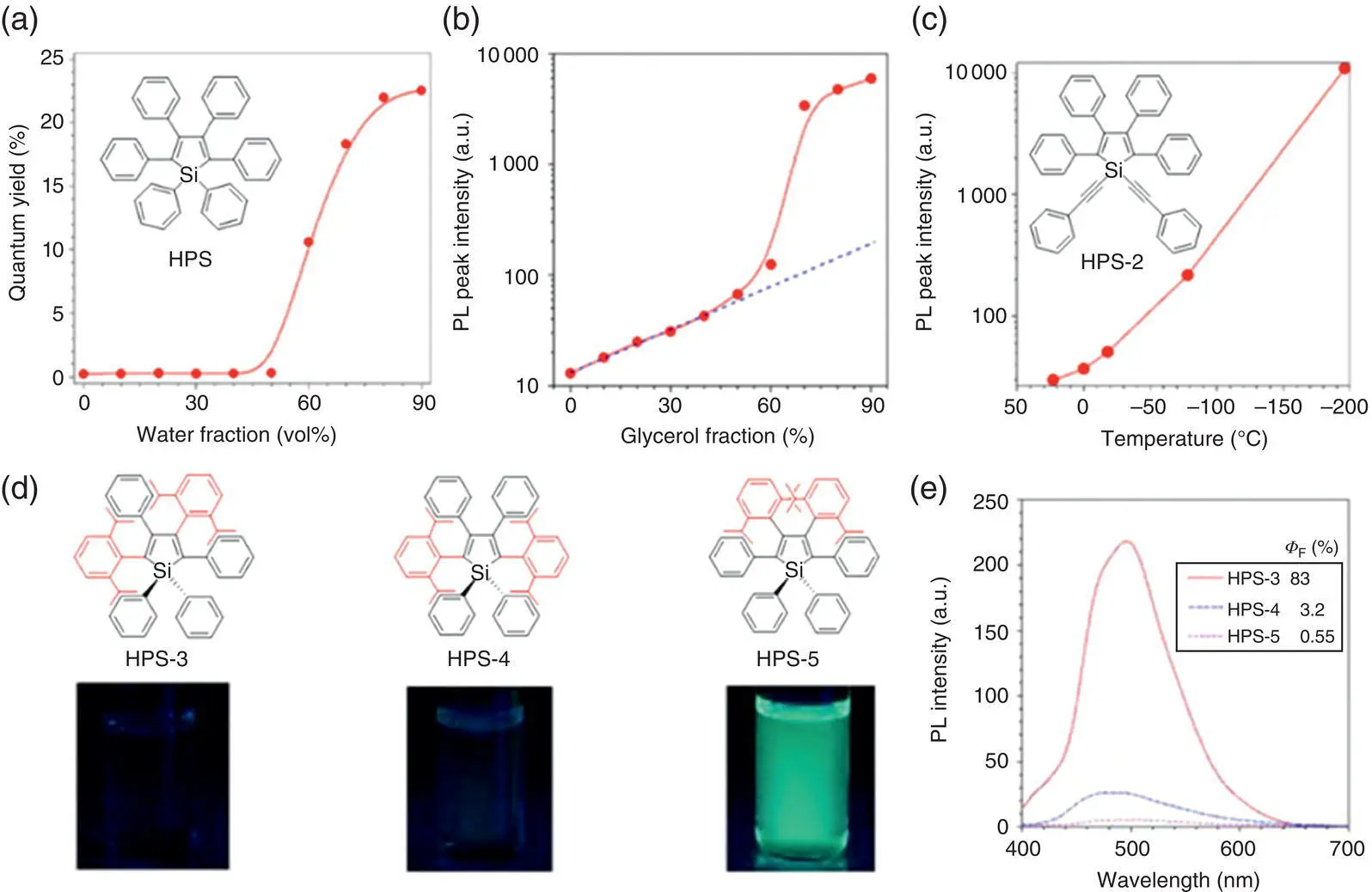
Figure 1.1 (a) Fluorescence quantum yield of HPS in the acetone/water mixtures with different water fraction. (b) Photoluminescence peak intensity of HPS in the glycerol/methanol mixtures with different glycerol fraction [HPS] = 10 −5M. (c) Photoluminescence peak intensity of HPS‐2 in the 1,4‐dioxane solution at different temperatures. [HPS‐2] = 10 −5M.
Source: Adapted from Ref. [14a] with permission from American Chemical Society.
(d) Chemical structures and fluorescence photos. (e) Photoluminescence spectra of HPS 3−5 in the acetone solution (10 −5M).
Source: Adapted from Ref. [14c] with permission from American Chemical Society.
TPE is another prototype of AIEgens with a similar intrinsic structure as HPS. Four phenyl rings are connected with the central double bond in a highly twisted conformation. As presented in Figure 1.3a, upon excitation, four phenyl rings can drastically twist against the double bond. Meanwhile, the C=C double bond will be weakened due to the photoexcitation, which endows it with strong twisting ability to reorganize the conformation. All these intramolecular motions result in the undetectable emission for TPE in the dilute solution. However, its emission can be dramatically boosted once the aggregation occurs. Further locking the adjacent phenyl rings through chemical bonds or attaching bulky groups onto the phenyl rings of TPE, its emission can be enhanced even in the solution [16]. All these experiments of controlling the internal or external constraints to the intramolecular motions have proved that the RIR mechanism is responsible for the AIE effect of the rotor‐based AIEgens.
1.2.2 Restriction of Intramolecular Vibration
Apart from the intramolecular rotation, the intramolecular vibration in the luminogens‐lacking rotors can also cause emission quenching in the dilute solution. Bu et al. have found that two coumarin derivatives fused with five‐membered and seven‐membered alkyl rings, respectively, show totally different photophysical behaviors, despite their similar conjugation degrees in the ground state, as indicated by the similar ultraviolet (UV)–vis absorption spectra presented in Figure 1.2[10a].
The compound CD‐5 with a five‐membered ring is much more rigid and can show strong light emission in the dilute solution, but its emission is weakened in the solid, whereas the CD‐7 is almost nonemissive in the dilute solution, but it can show enhanced light emission in the solid state. The calculated conformations of these two derivatives have revealed that the CD‐7 with seven‐membered ring owns much higher vibrational flexibility in the excited state. The central π ‐conjugated plane of CD‐7 will suffer the vibrational motion with a dihedral angle of around 18°, so its emission can be easily quenched in the dilute solution. But such intramolecular vibration can be efficiently restricted in the solid state, which finally leads to the AIE phenomenon. The mechanism for such AIEgens‐lacking rotors has been concluded as the RIV.
However, it remains unclear what the driving force is for the vigorous molecular motions on the excited state. Zhao et al. have recently systematically investigated the structure–property relationship of a series of annulene‐based AIEgens and figured out the driving force of the strong vibrations in the excited state [10]. The parent cyclooctatetrathiophene (COTh) contains an eight‐membered ring fused by four thiophene rings and takes a noncoplanar and saddle‐like conformation as presented in Figure 1.3b. Such a highly twisted structure makes COTh nonaromatic in the ground state. The COTh shows typical AIE behavior that it can only emit negligible light in the dilute solution but shows enhanced green emission in the aggregate and solid film. The optimized structure of COTh in the ground state is similar to its single crystal structure, whereas it can relax to a transition state with a planar conformation, and finally decay through fast nonradiative pathways to the ground state. Through the calculation of the nucleus‐independent chemical shift (NICS) and anisotropy of the induced current density (ACID) to characterizing the aromaticity of the structures, it has been revealed that the aromaticity reversal from the ground state to the excited state drives the conformational change through intense up–down vibration. But when the aggregation occurs, the vibration can be effectively restricted due to the environmental hindrance. Meanwhile, the energy of the planar transition state has been elevated, and the pathways to the transition state are not energetically accessible, which leads to the major radiative decay and promoted luminescence efficiency.
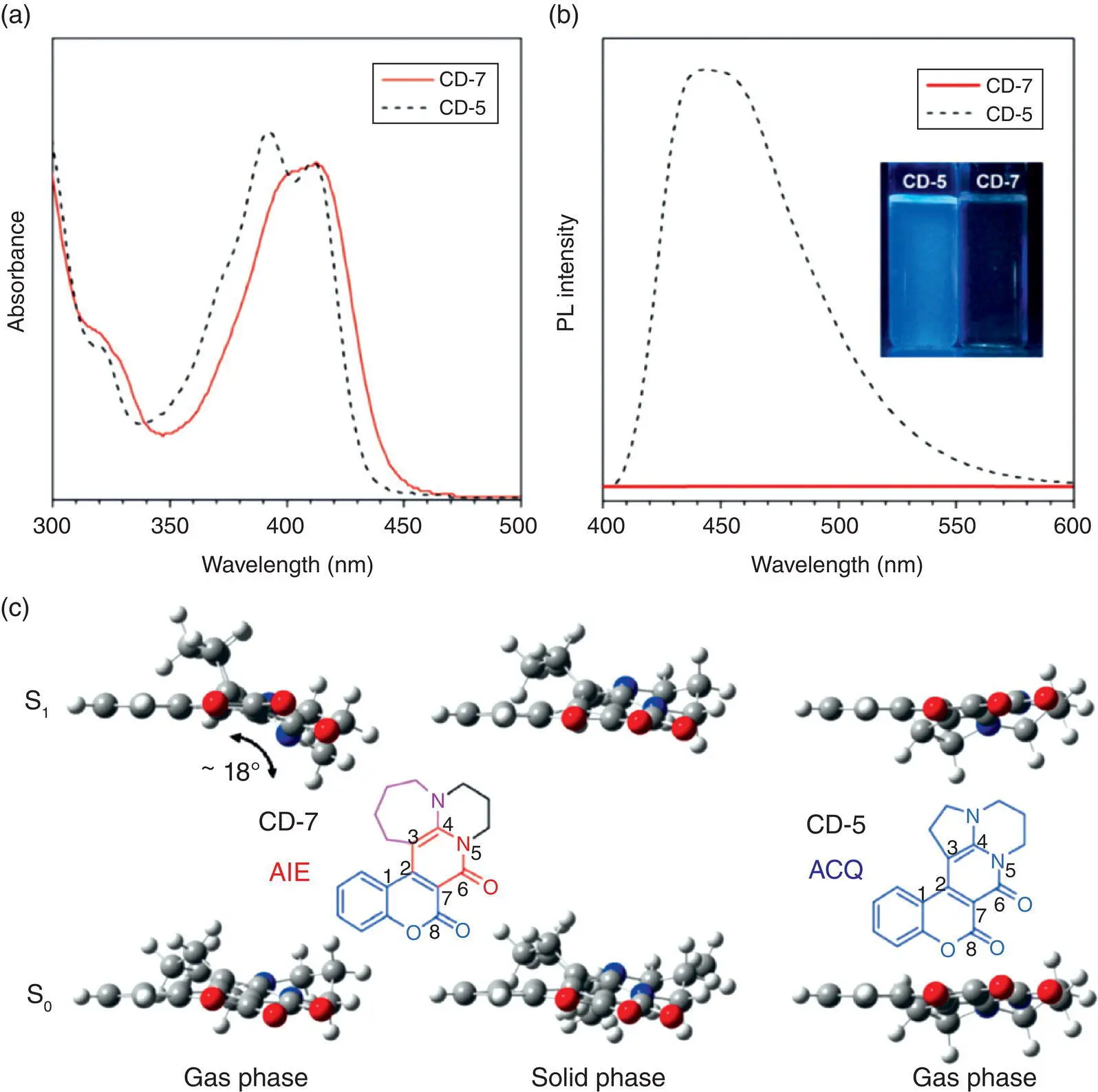
Figure 1.2 (a) UV–vis absorption and (b) photoluminescence (PL) spectra of coumarin derivative‐7 (CD‐7) and coumarin derivative‐5 (CD‐5) in CH 2Cl 2. Concentration: 10 −5M; excitation wavelength: 411 nm. (c) Optimized structures at the S 0,minand S 1,minof CD‐7 in the gas and solid phases and CD‐5 in the gas phase at time dependent (TD) M062X/6‐31G(d) level.
Source: Adapted from Ref. [10a] with permission from John Wiley and Sons.
From the abovementioned cases, it can be concluded that RIR and RIV are the main cause of the AIE properties of propeller‐shaped and shell‐shaped AIEgens. Hence, the RIM can be unified as the general working principle for the AIE effect, and RIM can serve as the basic design principle for the development of AIE materials.
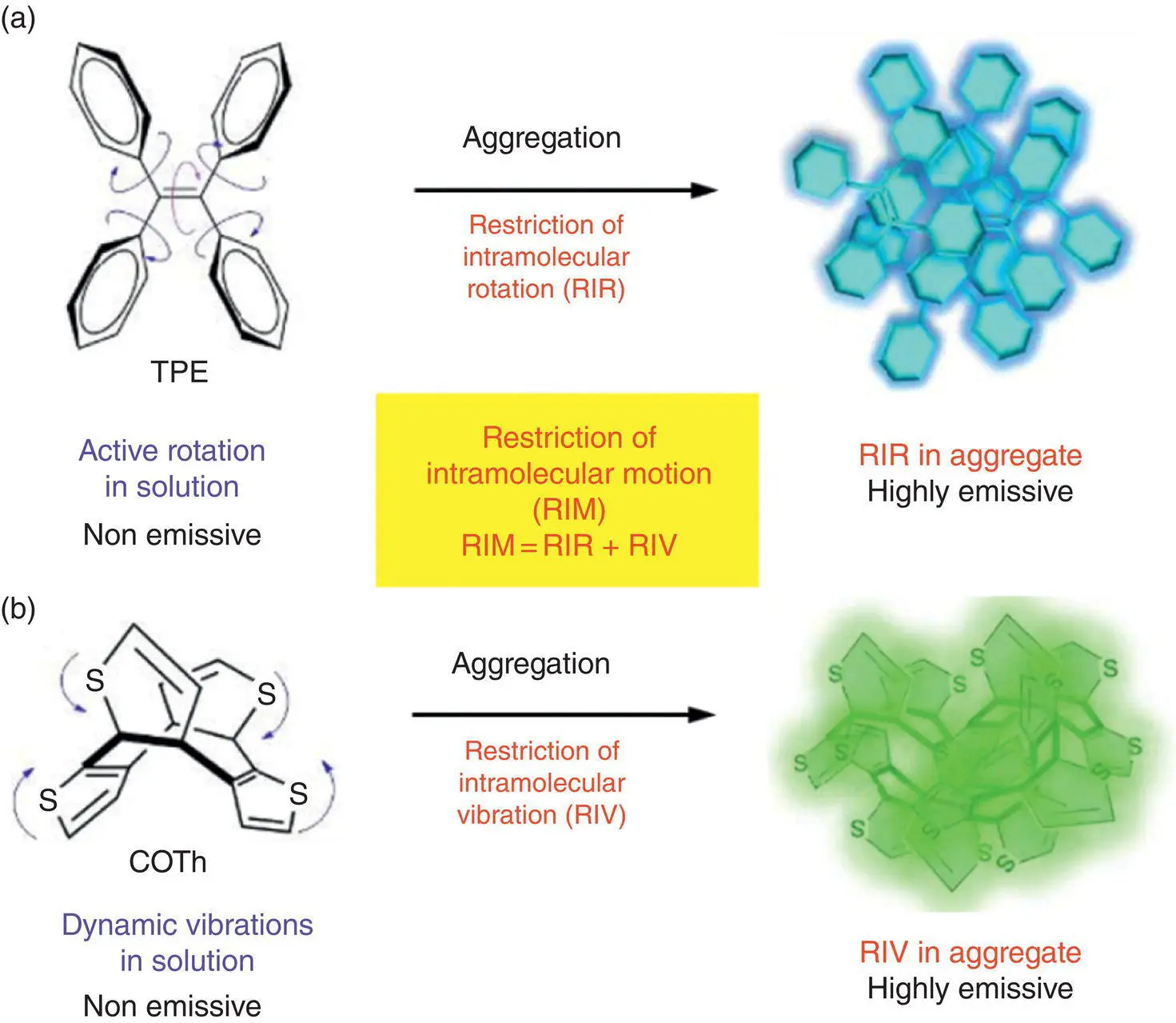
Figure 1.3 Schematic illustration of RIM mechanism. (a) Restriction of intramolecular rotation. (b) Restriction of intramolecular vibration.
Source: Adapted from Ref. [6a] with permission from American Chemical Society and Ref. [10b] with permission from Springer Nature.
1.2.3 Ultrafast Insights into Tetraphenylethylene Derivatives
Molecular motions significantly affect the decay behaviors for AIEgens. Certain excited‐state dynamics require further clarification. Tang and coworkers have developed detailed understanding of excited‐state molecular motions of TPE derivatives and further elucidated the RIM mechanism through the lens of ultrafast spectroscopy [16]. As presented in Figure 1.4, six TPE derivatives with different internal constraints and structural rigidity have been prepared and studied through the first‐principle calculation based on (time‐dependent) density function theory ((TD)DFT) methods.
Different structural rigidities may lead to different freedom of motion and structural variation during the excited‐state decay. Optimized conformations in the S 0state and the S 1state show that all the studied TPE derivatives undergo the elongation of the ethylenic double bond and form the quasi‐double bond after excitation, but the single bonds connecting the peripheral phenyl rings with the central double bond have been shortened. These two types of bond change originate from the weakening of the double bond and the electron density flowing from the double bond to the surrounding single bonds. Meanwhile, the bond variation leads to the twisting of the quasi‐double bond and the phenyl rotors.
Comparison between the S 1and S 0conformations shows that the parent TPE has encountered the largest structural reorganization with the torsion angle of the quasi‐double bond of 56.80° and the torsion angle of the phenyl ring of 29.04°, whereas with decreasing the motion freedom, derivatives show decreased structural changes. The TPE‐6 with double vicinal locking of the phenyl rings only shows the double bond elongation of 0.03 Å and less than 9° twisting of the quasi‐double bond. Hence, the twisting of the quasi‐double bond and the torsion of the phenyl rings have been chosen as the motion coordinates to scan the potential energy surfaces (PES) of the S 1and S 0states. The calculated minimum energy path (MEP) in the ground state of the parent TPE is coupled by the phenyl‐ring torsion from 50° to 90° and the double bond twisting of less than 9° with energy change of less than 7 kcal/mol. There is a transition state connecting two minimum points on the ground‐state PES with the highly twisted quasi double bond. However, the MEP on the excited‐state PES is dominantly associated with the twisting of the quasi‐double bond of 50° due to the significant elongation of the original double bond of 0.12 Å, accompanied by the phenyl ring’s torsion of less than 25°. The PES around the S 1,minis shallow with the energy of 60 kcal/mol, which is rather close to the transition state on the ground‐state PES, which will lead to fast decay from S 1,minto the ground state.
Читать дальше















