1 ...8 9 10 12 13 14 ...40 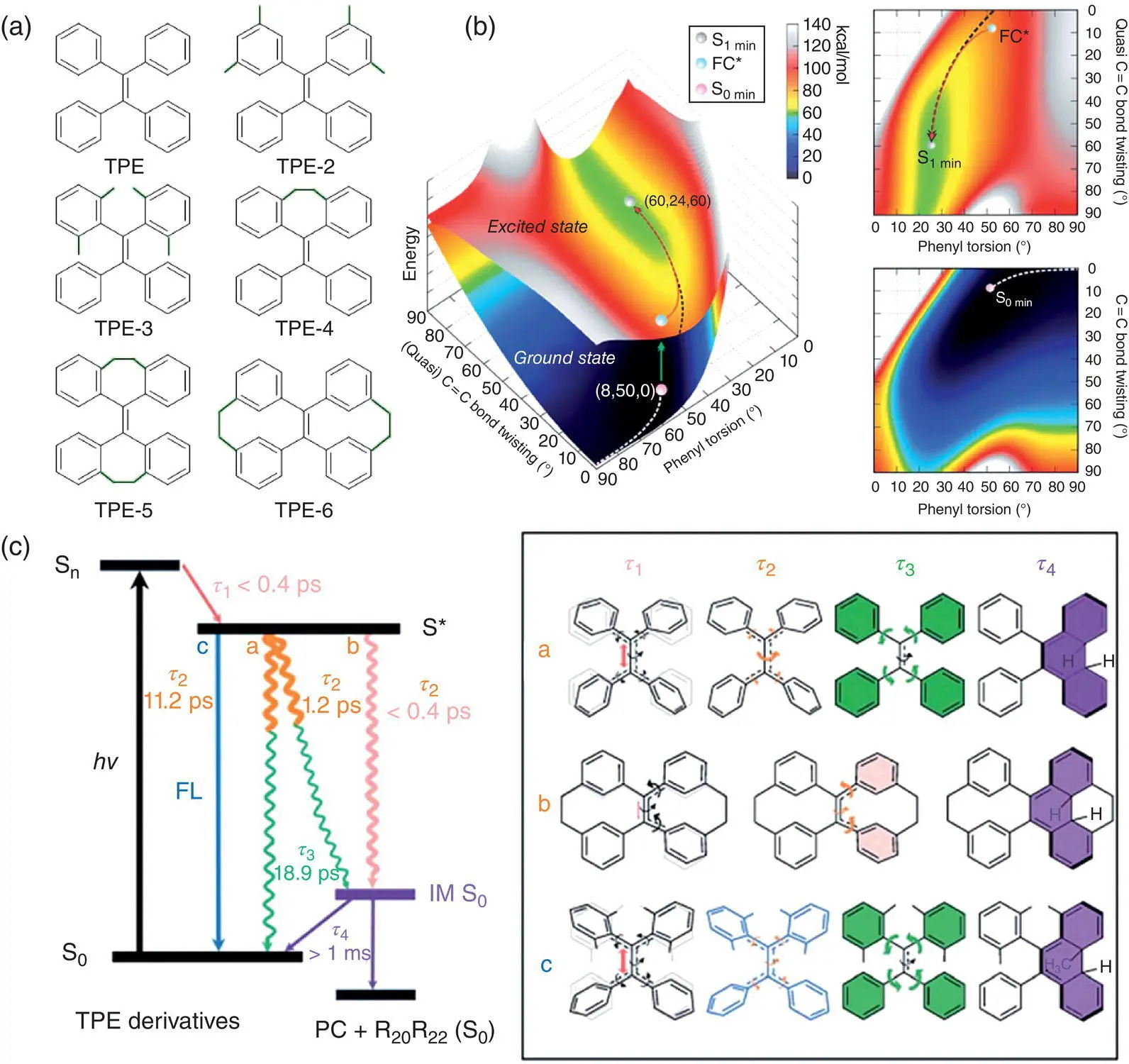
Figure 1.4 (a) Chemical structures of TPE derivatives with increasing rigidity. (b) Potential energy surfaces (PES) and projections of PES of the S 1and the S 0state of TPE. The MEPs on the S 1and the S 0state are marked as black and white dash line, respectively. (c) Schematic illustration of ultrafast excited‐state decay processes of TPE derivatives and dominant molecules with different timescales.
Source: Adapted from Ref. [16] with permission from The Royal Society of Chemistry.
The ultrafast transient absorption spectra have further revealed the excited‐state decay dynamics through the coupling of the double bond twisting and phenyl ring’s torsion. For the parent TPE, upon absorption of pump light, the excited species will decay from S nstates to the S 1state, through internal conversion within sub‐picosecond, mainly associated with elongation and twisting of the C=C double bond, which can be signalized by the stimulated emission at 1.3 ps. Then, from 1.3 to 3.79 ps, the excitons populated on the emissive state revert back through twisting the quasi‐double bond. After 3.79 ps, the photocyclization intermediate forms associated with the phenyl ring’s torsion with an ultralong lifetime of 159 seconds. With increasing the intramolecular steric congests, TPE‐2 shows similar excited‐state dynamics with the parent TPE, whereas TPE‐3 emits light efficiently within sub‐picosecond even in the dilute solution. The transient emission at 480 nm of TPE‐3 within 1.19 ps has confirmed the ultrafast population on the emissive S 1,min, which is consistent with the decrease of the peak at 477 nm on the ultrafast transient absorption spectra due to the stimulated emission between 0.63 and 1.2 ps. The population period takes a longer time as compared to the parent TPE, which indicates that the steric hindrance on the phenyl rings can slow down the twisting of the quasi‐double bond and contribute to the stabilization of the emissive state. Further attaching the alkyl tethers between the geminal or vicinal phenyl rings will largely reduce freedom of motion of the phenyl rings and accelerate the formation of the photocyclization intermediate.
Hence, it can be concluded that TPE derivatives will undergo an ultrafast vibrational relaxation and populate on the S 1,minafter excitation through the coupled molecular motions of elongation and twisting of the C=C double bond as well as torsion of phenyl rings within the picosecond timescale, after which the photocyclization intermediate will be formed. The dominant time component in this relaxation varies, depending on the conformational rigidity. The increased steric hindrance or structural locking on the parent TPE will hamper the twisting of the quasi‐double bond and stabilize the emissive state to generate efficient light, as in the case of TPE‐3, or directly minimize the motions involving the double bond and the phenyl rings and make the photocyclization intermediate formed on the sub‐picosecond timescale as in the case of TPE‐6. Thus, motions involving the C=C double bond and the formation of the photocyclization intermediate are the dominant pathways for the excited‐state deactivation of TPE derivatives in the solution.
1.2.4 Theoretical Insights into Restriction of Intramolecular Motion
The experimental investigation has proved that the intramolecular motions are responsible for the weak emission of AIEgens in the solution, and the aggregate environment can restrict such molecular motions and enhance the light emission. What kind of molecular motions can govern the excited‐state decay pathways for AIEgens? How do the molecular motions dissipate the excited‐state energy? Theoretical researchers have developed the proper modes for radiative decay rates and nonradiative decay rates based on the thermal vibration correlation function (TVCF) formalism, and precisely calculated the quantum yields in both solution state and aggregate state and finally revealed the quantum origins of the RIM mechanism [7].
Studies of the transition process between two electronic states relate to the quantum dynamics. The fluorescence and phosphorescence processes usually occur in the timescale from nanoseconds to milliseconds, while the internal conversion and intersystem crossing often occur in picoseconds or a shorter timescale. However, the current excited‐state dynamic simulation can only deal with the process occurring within picoseconds, which is much faster than the luminescence process. Hence, theoreticians usually evaluate the luminescence quantum efficiency by employing the decay rate formalism based on the Fermi golden rule [3]. The luminescence quantum yield Φ Fcan be defined as Φ F= k r+ k nr, in which k rstands for the radiative decay rate and k nrstands for the nonradiative decay rate. The k rcan be calculated with integration of multiple theories even for polyatomic molecular systems. The problem to evaluate the quantum yield is to precisely calculate the nonradiative decay rates, which contain more factors related to complicated molecular motion modes [3].
Traditional target molecules for the photophysical research always own highly rigid π ‐electron structure with large conjugation, which makes the effect of molecular motions less notable, whereas most AIEgens possess highly flexible molecular structures, allowing the high freedom of motion in the isolated state, which facilitates the nonradiative pathways to a large extent through diverse decay modes. Peng et al. have proved that calculation of internal conversion rates considering the Duschinsky rotation effect (DRE) leads to more accurate quantitative evaluation of the nonradiative decay process for the 1,2,3,4‐tetraphenylbutadiene (TPBD) with AIE property since DRE describes the coupling among the multiple modes of molecular motions [7e]. Such mode mixing also significantly contributes to the nonradiative dissipation of excited‐state energy as well as the intrinsic multiple molecular motions. On the other hand, the evaluation of the temperature‐dependent luminescence behaviors of TPBD considering DRE produces more reliable results that are much closer to the experimental observation [7e].
Furthermore, for the fluorescence process, the nonradiative decay pathways are governed by the nonadiabatic coupling (NAC), which can be divided into nonadiabatic electronic coupling (NAEC) and EVC [7e]. The NAEC deciphers the electronic part that contributes to the nonradiative decay, and the EVC relates to the interaction between the electronic and the nuclear motions. The reorganization energy (RE) can be applied to evaluate the intensity of EVC and can offer detailed information on the structure–property relationship [17]. Through investigating NAEC and EVC, researchers have revealed the quantum origins of the energy‐consuming mechanism from molecular motions. Taking HPS as the research model, its fluorescence quantum yield is only 0.30% in the dilute solution, whereas it can reach 78% in the solid film, which is 260 times as high as that in the solution. Zhang et al. have calculated the electronic structures of HPS in both isolated state and crystal state, using the combined quantum mechanics and molecular mechanics (QM/MM) method to explore the detailed working mechanism of HPS from quantum view [7a].
During the vibrational relaxation in the excited state, large conformational reorganization occurs, which mainly relates to the torsion of phenyl rotors on the 2,5‐positions of the silole ring due to their high mobility in the isolated state. However, the optimized HPS in the crystal shows only minor conformational reorganization during the relaxation with almost no change in the dihedral angles of phenyl rings, which proves the RIM features in the solid state. Further, NAC analysis reveals that the distribution and total values of NAEC have no notable change before and after the aggregation occurs ( Figure 1.5), indicating that the NAEC is insensitive to the aggregate environment. Nevertheless, the EVC analysis shows that many low‐frequency motion modes ( ω < 200 cm −1) of HPS exist in the isolated state, and they are mainly recognized as the torsion of phenyl rotors. The RE of these low‐frequency modes is 197 meV, which occupies 40% of the total RE of the whole system. Afterwards, the RE of low‐frequency modes significantly decreases to 84 meV in the crystal, occupying 21% of the total value. Due to the large suppression of EVC, the calculated k nrdecreases with four orders in magnitude from the isolated state to the crystal state. Hence, the emission efficiency of HPS can be boosted in the crystal state due to the restricted EVC.
Читать дальше













