When taking the dynamic evolution along the PES into consideration, it can be found that the TPE derivatives also have abundant decay pathways in the excited state, including both photophysical processes and photochemical reactions [8]. The excited‐state molecular dynamics simulation for 1.5 ps based on the trajectory surface hopping (TSH) model shows that 75% of the simulated trajectories decay to the ground state through a CI and form the cyclization product with a new C–C single bond between the two adjacent phenyl rings. The distance between the two carbons is only 2.0 Å at the cyclization CI. There is 5% of the trajectory decay through the twisting of the C=C double bond. Similar decay features have been found in two TPE derivatives substituted with methyl groups at meta and ortho positions. The scanning of the linear interpolation in internal coordinates (LIIC) path using the OM2/multireference configuration interaction (MRCI) method is presented in Figures 1.6d and 1.7c.
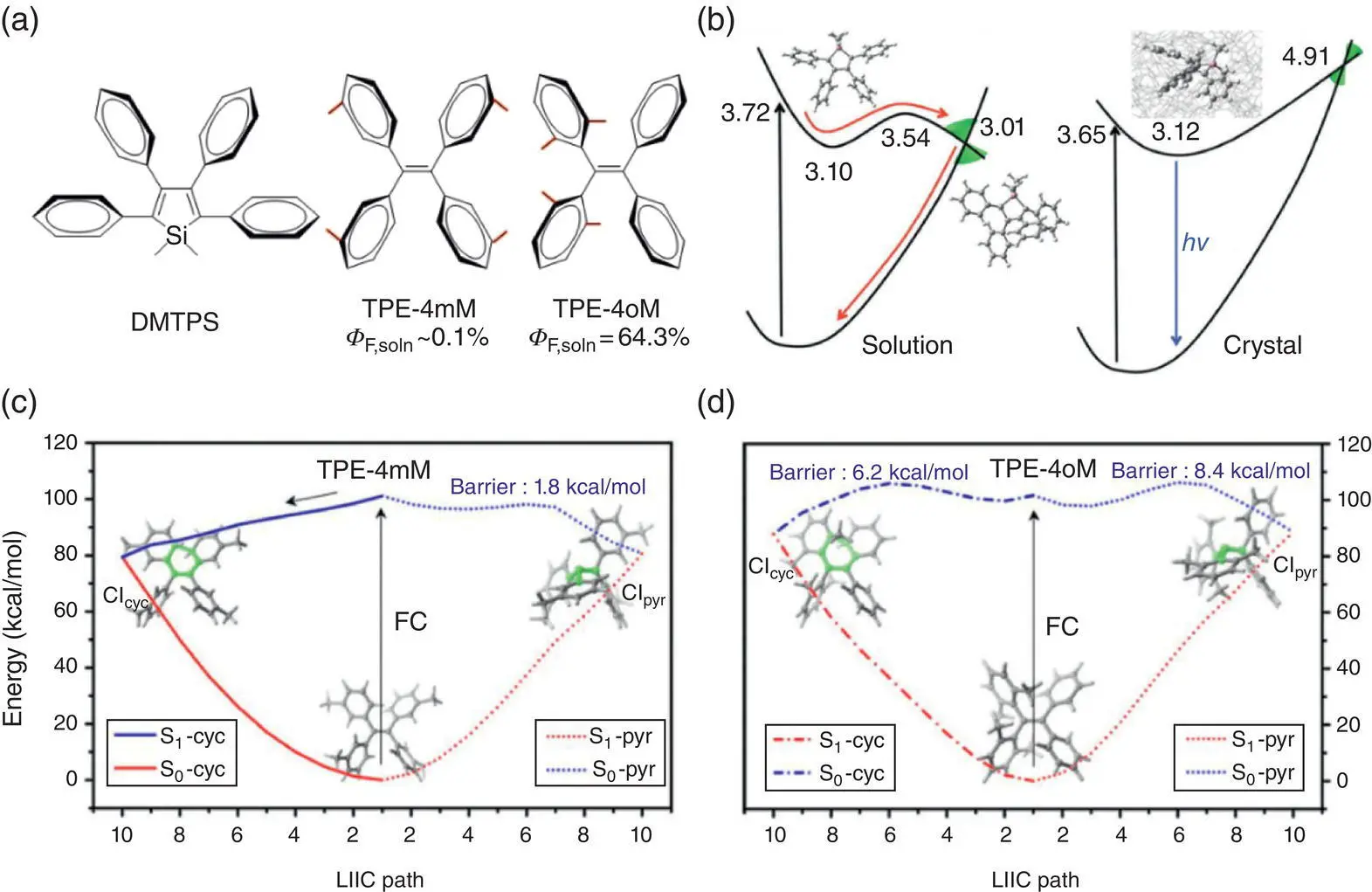
Figure 1.7 (a) Chemical structures and fluorescence quantum yield in the dilute solution of DMTPS, TPE‐4mM, and TPE‐4oM. (b) Schematic illustration of the potential energy profiles of DMTPS in the cyclohexane and the crystal. The potential energy profile in the cyclohexane is optimized at the CASSCF/6‐31G (d) level with the PCM model. The vertical excitation energy for each point is recalculated using CASPT2 with the ANO‐S basis set contracted to Si[4s3p1d]/C[3s2p1d]/H[2s1p]. The calculation in the crystal is performed by using ONIOM(CASSCF/6‐31G(d):UFF) model with recalculating the QM energy by CASPT2.
Source: Adapted from Ref. [18b] with permission from The Royal Society of Chemistry.
Linear interpolation in internal coordinates paths calculated by using the OM2/MRCI method of (c) TPE‐4mM and (d) TPE‐4oM.
Source: Adapted from Ref. [8a] with permission from American Chemical Society.
Upon photoexcitation, TPE‐4 mM can decay through the CI of cyclization with a negligible barrier, which is consistent with the TSH simulation that 88% of the total 558 trajectories drops into the ground state along the cyclization direction. On the other hand, there is only a relatively small barrier of 1.8 kcal/mol between the FC region and the CI for E / Z isomerization, which indicates TPE‐4mM will undergo ultrafast nonradiative decay so that it is almost nonemissive in the solution. On the contrary, for the TPE‐4oM with the methyl groups substituted at the ortho position of the phenyl rings, there are two notable barriers on both the cyclization and isomerization pathways, connecting the FC region with the cyclization CI and the isomerization CI, respectively. Hence, there is no decay for TPE‐4oM to the ground state of the total 568 trajectories in the TSH simulation for 1 ps, which accounts for its higher emission efficiency in the solution.
With scanning the PES or simulating by the TSH method, we can find that vigorous molecular motions of the highly flexible AIEgens in the excited state can lead to the CIs between the excited state and the ground state. These CIs will result in enormous nonradiative decay rates or generate another photoinduced product. Furthermore, the crossing between the excited states can also strongly affect the photophysical behaviors of luminogens that will generate the transition forbidden dark states.
1.4 Restriction of Access to the Dark State
RIM serves as the most effective guideline for the design of AIE molecules. However, RIM requires a more detailed elaboration when it is applied to the heteroatom‐containing AIEgens. On the one hand, the introduction of heteroatoms endows the luminogens versatile functions, whereas, on the other hand, it makes the excited‐state features of the luminogens more complicated. First, introduction of heteroatoms, often, produces the electron‐donating or ‐withdrawing effect, and then facilitates the mixing of the overlap‐forbidden charge‐transfer (CT) state with the local‐excited (LE) state. Second, heteroatoms with lone‐pair electrons can import the crossing between the overlap‐forbidden ( n , π *) state with the ( π , π *) state. What is more, ( n , π *) state will facilitate the intersystem crossing from the singlet states to the triplet states, which are spin forbidden and can be easily quenched. All these overlap‐forbidden and spin‐forbidden states have been defined as dark states that are detrimental to the fluorescence process [19].
Taking the (9‐anthrylmethyl) bis(2‐pyridylmethyl) amine (APA) as an example ( Figure 1.8) [9], the APA only contains a large π ‐conjugation plane consisting of the anthracene and a triple‐pyridine motif, but it shows the unique AIE property, which differs from typical AIEgens, usually, with multiple rotors. Although the APA contains the anthracene with large conjugation, it shows weak UV emission with a Φ Fof 0.6% in the dilute tetrahydrofuran (THF) solution with vibronic peaks. After chelation with the zinc ion, it shows enhanced emission with the Φ Fof about 100% with the similar vibronic peaks. Meanwhile, with more than 90% water added into the pure solution of APA, the resulting APA aggregate shows a broad and red‐shifted emission band with a peak at 461 nm. The APA crystal also shows a similar emission band at 492 nm. The large spectral variation between the dilute solution and the solid state indicates the mixing of excited states with different characteristics.
Indeed, with optimization of the first two excited states of the isolated APA involving the transition of the lone pair electrons, two kinds of excited‐state features have been figured out. According to the transition characteristics, the two close‐lying excited states are assigned as ( π , π *) state and ( n , π *) state, which are vibronically coupled by the molecular motions. The bright ( π , π *) min owns large oscillator strength and is responsible for the light emission, whereas the lower‐lying ( n , π *) min has the oscillator strength nearly close to 0 and crosses the ( π , π *) state through the CI, which causes the quenching of the light emission. The key molecular motion connecting these two steady excited‐state points is the rotating of the nitrogen‐containing group. Hence, when chelating with the zinc ion, the lone pair electrons of the nitrogen atoms and the motion of the whole chelating group are blocked, which results in the elevation of the ( n , π *) state and recovering of the light emission from the bright ( π , π *) state. Similarly, the APA molecules in the crystal can pack tightly to form dimers and stabilized by the intermolecular π – π interaction and multiple C–H interactions, which effectively hinder the rotation of the chelating groups. The lock of lone‐pair electrons and motions of the nitrogen‐containing group lead to the elevation of the dark ( n , π *) state and finally boost the fluorescence from the bright ( π , π *) state.
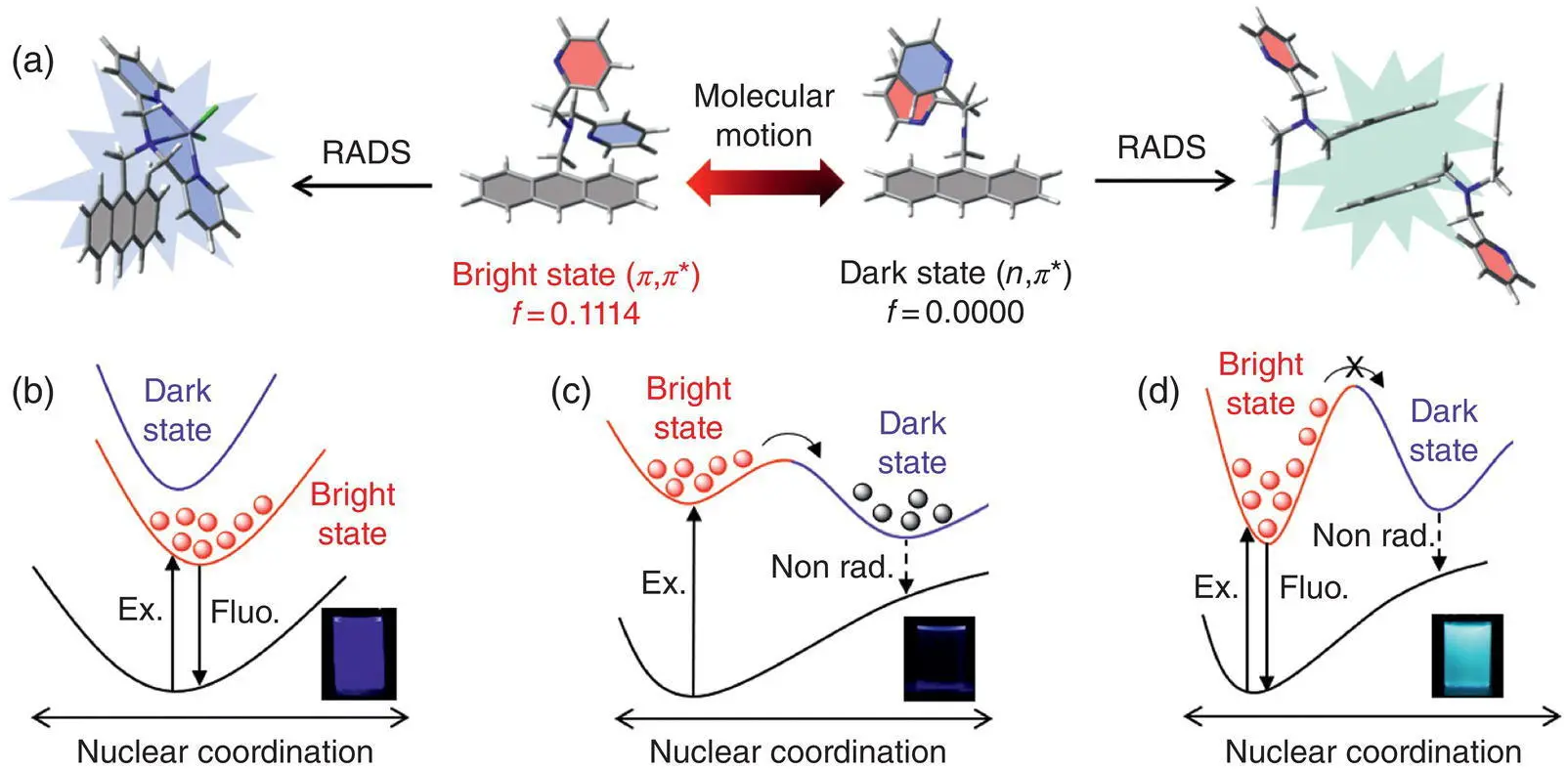
Figure 1.8 (a) Illustration of the coupling between the bright state and the dark state by the molecular motion, and the structures at the minimum points of the bright state and the dark state, the emissive chelation complex, and the dimer in the crystal. Schematic potential energy surfaces of RADS related to (b) the chelation with the zinc ion, (c) the dilute solution state, and (d) the dimers in the crystal.
Читать дальше














