Such nonconventional phenomenon has been coined as CTE; it deciphers that the severe molecular motions and nonconjugated structures make the CTE luminogens nonemissive in the isolated state or the dilute solution, whereas diverse intra‐ and intermolecular interactions in the cluster or cross‐linking can hamper the molecular motions and stabilize the conformations with large electron overlapping and thus boost the light emission of CTE luminogens, in which the packing density and the cluster size can affect the emission efficiency [21]. Taking the polyacrylonitrile (PAN) [21b] and poly( N ‐hydroxysuccinimidyl methacrylate) (PNHSMA) [21c] as the example, they contain no aromatic groups and are nonconjugated in the isolated polymer backbone ( Figure 1.10c). Therefore, these two molecules are nearly nonemissive in the dilute solution. But they can emit strong blue light in the concentrated solution or solid powder. CTE phenomenon also exists in the natural products such as starch, cellulose, and protein; they can show notable blue light in the solid state under UV excitation [21d].
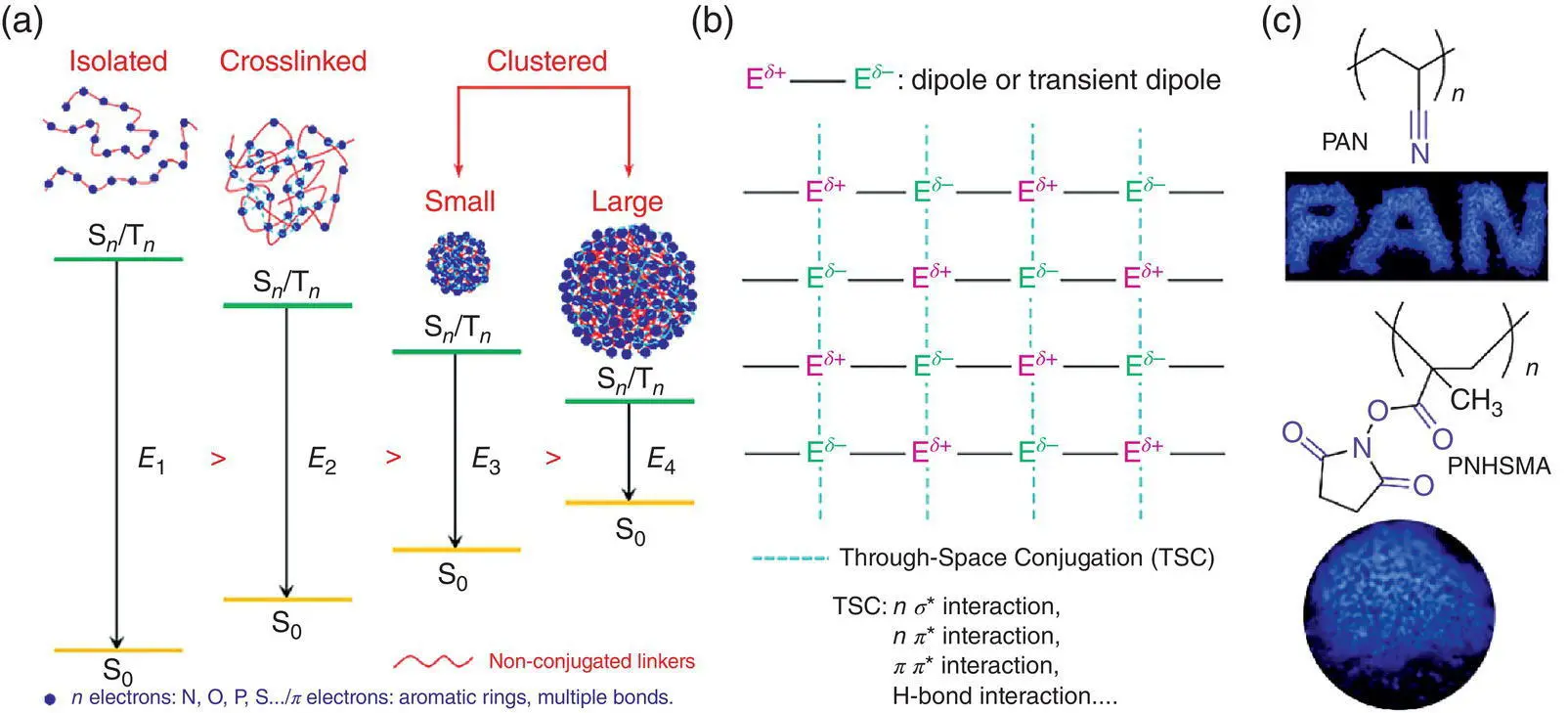
Figure 1.10 (a) Schematic illustration of the cluster formation, energy variation, and (b) the formation of through‐space conjugation involved in the CTE.
Source: Adapted from Ref. [21a] with permission from Elsevier.
(c) Examples of CTEgens.
Source: Adapted from Ref. [21b] with permission from John Wiley and Sons.
1.6.2 Polymerization‐induced Emission
Recently, Tang and coworkers have proposed polymerization‐induced emission (PIE), which is another conceptual innovation related to CTE [22]. It describes the process where the nonemissive monomers can be converted into luminescent polymers through polymerization. AIE process occurs mainly by physically manipulating the molecular motions, whereas PIE is achieved through the chemical ways accompanied by the CTE process. As versatile polymerization methods can be utilized to construct the PIE polymers, and these unusual luminescent polymers own good processability, the PIE acts as a promising solution in developing novel soft luminescence materials.
The working principle underneath is similar with CTE, nonconjugated subunits with rich electrons, such as phenyl, hydroxyl, and carbonyl groups, ether, and amide, can be connected into polymer chains by chain polymerization or step polymerization, and such polymer chains can be entangled and form multilevel structures through diverse intra‐ or interchain motions. Then, the electron‐rich moieties will aggregate into a cluster with electron overlapping in multiple microstructures and finally generate visible light. The emission intensity of the PIE polymers will increase with promoting the polymerization degree and the molecular weight. The intrinsic diverse structures endow the PIE polymers the potential to create diverse luminescence performance.
1.6.3 Excited‐state Through‐space Conjugation
From the abovementioned discussion, molecular motions are mainly detrimental to luminescence. Tang and coworkers have recently revealed a new role of molecular motions in the excited‐state deactivation process of the tetraphenylethane ( s ‐TPE) derivatives as presented in Figure 1.11a and b [13a].
The s ‐TPE only contains four phenyl rings connected by the saturated single bond but can intensely emit visible light with the peak at 467 nm in the solid state. The dilute solution of s ‐TPE shows ultraviolet emission that basically stems from the isolated phenyl rings, but with adding more than 70% water into the dilute solution, s ‐TPE molecules can aggregate accompanied with a notable emission peak at 460 nm emerging, showing the typical AIE property. Why do the nonconjugated systems emit the visible light? Due to the highly twisted and flexible structure of s ‐TPE, it shows no obvious intermolecular π – π interaction in the single crystal. The theoretical simulation of the exciton coupling also shows that there is no notable intermolecular coupling in the excited state, so it should be the intramolecular interaction that affects the emission.
Further optimization of the excited‐state structures shows that s ‐TPE will encounter substantial conformational reorganization, and during the relaxation, the distance between the geminal phenyl rings can gradually decrease. When it decays to the S 1,min, two geminal phenyl rings reach a close‐contacting conformation with large through‐space overlapping of π orbitals. Indeed, the transition energy gaps decrease along with the phenyl rings getting close, so the emission wavelengths can be redshifted with such intramolecular motions. Finally, the calculated emission at S 1,minbecomes 460 nm, which is well consistent with the experimental data. The EVC analysis shows that s ‐TPE molecules own much higher excited‐state motion ability in the dilute solution, so the conformation with intramolecular TSC can be easily disturbed by the vigorous molecular motions. Once the s ‐TPE molecules aggregate, the molecular motions will be restricted to a certain degree, as indicated by the suppressed structural reorganization, which, thus, stabilizes the through‐space conjugated conformation and reduces the nonradiative decay rates, and so the solid‐state s ‐TPE can emit enhanced visible light.
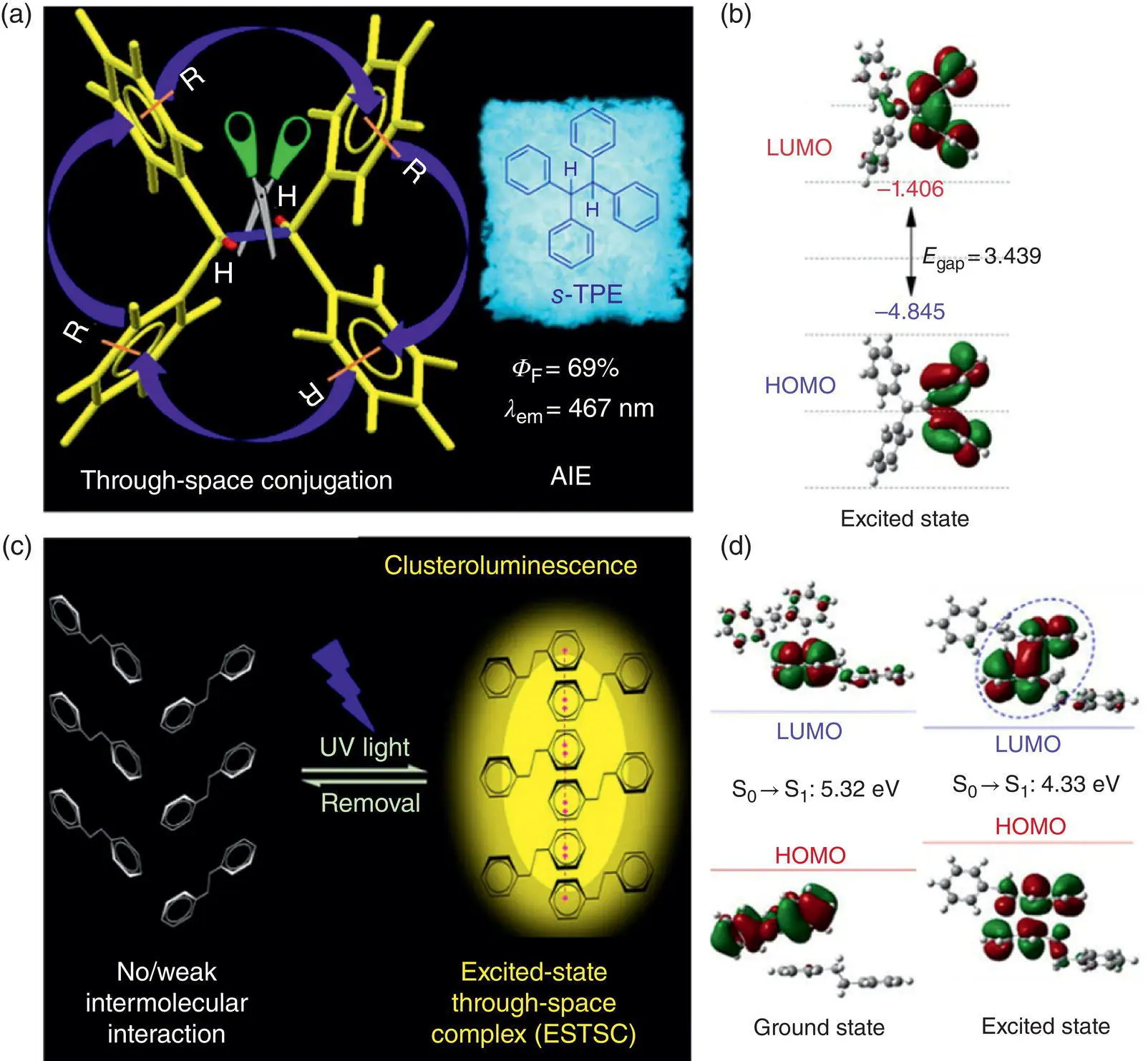
Figure 1.11 Schematic illustration of the excited‐state (a) intramolecular through‐space conjugation of s ‐TPE and (c) intermolecular through‐space complex of s ‐DPE. Molecular orbitals involved in the transition between the excited state and the ground state of (b) s ‐TPE and (d) s ‐DPE.
Source: Adapted from Refs. [13a, b] with permission from American Chemical Society.
A certain degree of molecular motions in the solid state will facilitate the formation and stabilization of emissive states with TSC. The diphenylethane ( s ‐DPE) is another nonconventional AIEgen as presented in Figure 1.11c and d [13b]. It contains no long‐range through‐bond conjugated structure but two isolated phenyl rings. The absorption and photoluminescence spectra in the dilute THF solution are ascribed to the electronic transition of the phenyl rings, with peaks at 270 and 285 nm, respectively. However, with addition of water into its dilute solution, a new emission peak at 355 nm gradually emerges, showing the AIE phenomenon. The emission peak in the solid state of s ‐DPE is also redshifted to 355 nm with a small fraction of the emission at 285 nm coming from the isolated state. Different from s ‐TPE, the isolated s ‐DPE molecule cannot form the intramolecular TSC, so the only way to redshift the light emission is to form the intermolecular TSC. The optimization of the dimer of s ‐DPE based on two adjacent s ‐DPE molecules in the single crystal shows that the two s ‐DPE molecules gradually get closer and turn the orientation of two phenyl rings from face‐to‐edge at S 0,minto face‐to‐face at S 1,minand finally form an excited‐state through‐space complex (ESTSC) with significant orbit overlapping between the two respective phenyl rings of the two s ‐DPE molecules. Meanwhile, the energy gap decreases from 5.32 to 4.33 eV along with the light‐driven solid‐state intermolecular motions, which is the luminescence origin of s ‐DPE in the solid state.
Читать дальше














