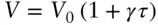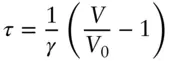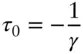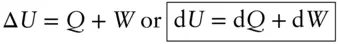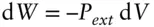(2.19) 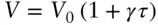
where V 0is the volume at some reference point where τ = 0 ( Figure 2.3a) and γ is a scale factor. For example, we might choose τ = 0 to be the freezing point of water and the scale factor such that γ = 100 ( Figure 2.3b) occurs at the boiling point of water, as is the case in the centigrade scale. Rearranging, we have:
(2.20) 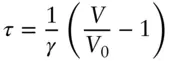
Then τ = 0 at V = V 0. If V is less than the reference volume, then temperature will be negative on our scale. But notice that while any positive value of temperature is possible on this scale, there is a limit to the range of possible negative values. This is because V can never be negative. The minimum value of temperature on this scale will occur when V is 0. This occurs at:
(2.21) 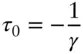
Thus, implicit in the ideal gas law, which we used to make this thermometer, is the idea that there is an absolute minimum value, or an absolute zero, of temperature, which occurs when the volume of an ideal gas is 0. Notice that while the value (−1/ γ ) of this absolute zero will depend on how we designed our thermometer (i.e., on V 0), the result, that a minimum value exists, does not. We should also point out that only an ideal gas can have a volume of 0. The molecules of real gases have a finite volume, and such a gas will have a finite volume at absolute zero.
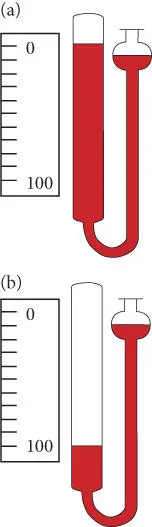
Figure 2.3 An ideal gas thermometer. The colored area is the volume occupied by the ideal gas.
The temperature scale used by convention in thermodynamics is the Kelvin ‡scale. The magnitude of units, called kelvins (not degrees kelvin) and designated K (not °K), on this scale are the same as the centigrade scale, so there are exactly 100 kelvins between the freezing and boiling points of water. There is some slight uncertainty (a very much smaller uncertainty than we need to concern ourselves with) concerning the value of absolute zero (i.e., the value of γ in eqns. 2.20and 2.21). The scale has been fixed by choosing 273.16 kelvins to be the triple point of water (0.01°C). On this scale, the absolute zero of temperature occurs at 0 ± 0.01 kelvins. The Kelvin scale should be used wherever temperature occurs in a thermodynamic equation .
Temperature has another fundamental property, and this is embodied in the zeroth law of thermodynamics . It is sufficiently obvious from everyday experience that we might overlook it. It concerns thermal equilibrium and may be stated in several ways: two bodies in thermal equilibrium have the same temperature and any two bodies in thermal equilibrium with a third are in equilibrium with each other .
2.5 ENERGY AND THE FIRST LAW OF THERMODYNAMICS
2.5.1 Energy
The first law may be stated in various ways:
Heat and work are equivalent §.
Energy is conserved in any transformation.
The change of energy of a system is independent of the path taken.
All are restatements of the law of conservation of energy:
Energy can be neither created nor destroyed.
Mathematically:
(2.22) 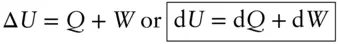
Thermodynamics is concerned only with the internal energy of a system. We don't really care whether the system as a whole is in motion, i.e., whether it has kinetic energy (we do care, however, about the internal kinetic energy, or heat). For the most part, we also don't care whether it has potential energy, except to the extent that this influences the state of our system (e.g., pressure in the atmosphere is a function of the altitude, and hence would be of interest to us). In addition, we are almost always concerned only with energy changes , not with the absolute energy of a system. In thermodynamics, Δ U , not U , is the interesting quantity.
Of course, we now understand that it is not energy that is conserved, but rather mass energy. Albert Einstein proposed this important modification of Joule's result in 1905 (Einstein, 1905). Conversion of mass to energy fuels the Sun and the stars and, through radioactive decay, is an important source of energy in the Earth. Radioactive decay and nuclear fusion will be important topics in Chapters 8and 10. For the geochemical processes we will be interested in the next few chapters, however, we can take the conservation of energy alone to be absolute.
Energy may be transferred between a system and its surroundings in several ways: heat, work, radiation, and advection (i.e., energy associated with mass gained or lost by the system). Whenever possible, we will want to choose our system such that it is closed and we don't have to worry about the latter. In most, but not all, instances of geochemical interest, radiation is not important. Thus in geochemical thermodynamics, heat and work are the forms of energy flow of primary interest .
We have seen that work is the integral of force applied through a distance. Force times distance has units of energy (mass-velocity 2), thus work is a form of energy. The SI ( Système Internationale ) unit of energy is the joule = 1 kg-m 2/s 2. Conversion factors for energy and other variables as well as values of important constants are listed in Appendix I.
There are several kinds of work of interest to thermodynamics, the most important of which is that involved in chemical reactions (later, when we consider oxidation and reduction reactions, we will be concerned with electrochemical work). One of the most important forms of work in classical thermodynamics is ‘PV’ work: expansion and contraction. Expressing eqn. 2.4in differential form:
(2.23) 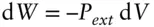
Pressure is force per unit area, and therefore has units of mass-distance –1-time –2, while volume has units of distance 3. The product of P and V therefore has units of energy: mass-(distance/time) 2. *The negative sign arises because, by convention, we define energy flowing into the system as positive. Work done by the system is thus negative, while work done on the system is positive. This conforms to a 1970 IUPAC (International Union of Pure and Applied Chemistry) recommendation.
While PV work is not as important in geochemistry as in other applications of thermodynamics, it is nevertheless of significant interest. There is, of course, a great range of pressures within the Earth.
Systems rising within the Earth, such as magma, a hydrothermal fluid, or upwelling water in the ocean or air in the atmosphere, will thus do work on their surroundings, and systems sinking, such as sediments being buried or lithosphere being subducted, will have work done on them.
We mentioned the concept of reversible and irreversible reactions and stated that a reversible reaction is one that occurs in sufficiently small steps that equilibrium is maintained. In an expansion or contraction reaction, equilibrium is maintained, and the reaction is reversible if the external pressure is equal to the internal pressure. The work done under these conditions is said to be reversible:
Читать дальше