A system may be related to its environment in a number of ways. An isolated system can exchange neither energy (heat or work) nor matter with its surroundings. A truly isolated system does not exist in nature, so this is strictly a theoretical concept. An adiabatic system can exchange energy in the form of work, but not heat or matter, with its surroundings, that is to say it has thermally insulating boundaries. Though a truly adiabatic system is probably also a fiction, heat transport in many geologic systems is sufficiently slow that they may be considered adiabatic. Closed systems may exchange energy, in the form of both heat and work, with their surrounding but cannot exchange matter. An open system may exchange both matter and energy across it boundaries. The various possible relationships of a system to its environment are illustrated in Figure 2.1.
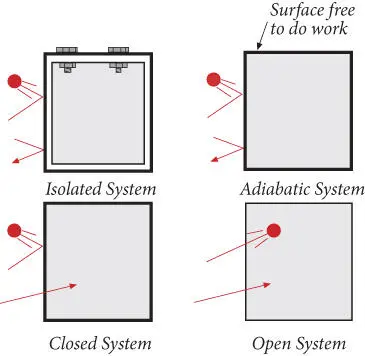
Figure 2.1 Systems in relation to their surroundings. The ball represents mass exchange, the arrow represents energy exchange.
Depending on how they behave over time, systems are said to be either in transient or time-invariant states. Transient states are those that change with time. Time-independent states may be either static or dynamic . A dynamic time-independent state, or steady-state , is one whose thermodynamic and chemical characteristics do not change with time despite internal changes or exchanges of mass and energy with its surroundings. As we will see, the ocean is a good example of a steady-state system. Despite a constant influx of water and salts from rivers and loss of salts and water to sediments and the atmosphere, its composition does not change with time (at least on geologically short time-scales). Thus, a steady-state system may also be an open system. We could define a static system as one in which nothing is happening. For example, an igneous rock or a flask of seawater (or some other solution) is static in the macroscopic perspective. From the statistical mechanical viewpoint, however, there is a constant reshuffling of atoms and electrons, but with no net changes. Thus, static states are generally also dynamic states when viewed on a sufficiently fine scale.
Let's now consider one of the most important concepts in physical chemistry, that of equilibrium . One of the characteristics of the equilibrium state is that it is static from a macroscopic perspective, that is, it does not change measurably with time. Thus, the equilibrium state is always time-invariant. However, while a reaction A→B may appear to have reached static equilibrium on a macroscopic scale, this reaction may still proceed on a microscopic scale but with the rate of reaction A→B being the same as that of B→A. Indeed, as we shall see in Chapter 5, a kinetic definition of equilibrium is that the forward and reverse rates of reaction are equal.
The equilibrium state is entirely independent of the manner or pathway in which equilibrium is achieved. Indeed, once equilibrium is achieved, no information about previous states of the system can be recovered from its thermodynamic properties. Thus a flask of CO 2produced by combustion of graphite cannot be distinguished from CO 2produced by combustion of diamond. In achieving a new equilibrium state, all records of past states are destroyed.
Time-invariance is a necessary but not sufficient condition for equilibrium. Many systems exist in metastable states. Diamond at the surface of the Earth is not in an equilibrium state, despite its time-invariance on geologic time-scales. Carbon exists in this metastable state because of kinetic barriers that inhibit transformation to graphite, the equilibrium state of pure carbon at the Earth's surface. Overcoming these kinetic barriers generally requires energy. If diamond is heated sufficiently, it will transform to graphite, or in the presence of sufficient oxygen, to CO 2.
The concept of equilibrium versus metastable or unstable (transient) states is illustrated in Figure 2.2by a ball on a hill. The equilibrium state is when the ball is in the valley at the bottom of the hill, because its gravitational potential energy is minimized in this position. When the ball is on a slope, it is in an unstable, or transient, state and will tend to roll down the hill. However, it may also become trapped in small depressions on the side of the hill, which represent metastable states. The small hill bordering the depression represents a kinetic barrier. This kinetic barrier can only be overcome when the ball acquires enough energy to roll up and over it. Lacking that energy, it will exist in the metastable state indefinitely.
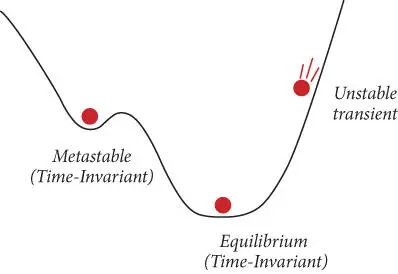
Figure 2.2 States of a system.
In Figure 2.2, the ball is at equilibrium when its (gravitational) potential energy is lowest (i.e., at the bottom of the hill). This is a good definition of equilibrium in this system, but as we will soon see, it is not adequate in all cases. A more general statement would be to say that the equilibrium state is the one toward which a system will change in the absence of constraints. So in this case, if we plane down the bump (remove a constraint), the ball rolls to the bottom of the hill. At the end of this chapter, we will be able to produce a thermodynamic definition of equilibrium based on the Gibbs free energy. We will find that, for a given pressure and temperature, the chemical equilibrium state occurs when the Gibbs free energy of the system is lowest.
Natural processes proceeding at a finite rate are irreversible under a given set of conditions; that is, they will only proceed in one direction. Here we encounter a problem in the application of thermodynamics: if a reaction is proceeding, then the system is out of equilibrium and thermodynamic analysis cannot be applied. This is one of the first of many paradoxes in thermodynamics. This limitation might at first seem fatal, but we get around it by imagining a comparable reversible reaction. Reversibility and local equilibrium are concepts that allow us to “cheat” and apply thermodynamics to nonequilibrium situations. A “reversible” process is an idealized one where the reaction proceeds in sufficiently small steps that it is in equilibrium at any given time (thus allowing the application of thermodynamics).
Local equilibrium embodies the concept that in a closed or open system, which may not be at equilibrium on the whole, small volumes of the system may nonetheless be at equilibrium. There are many such examples. In the example of a mineral crystallizing from magma, only the rim of the crystal may be in equilibrium with the melt. Information about the system may nevertheless be derived from the relationship of this rim to the surrounding magma. Local equilibrium is in a sense the spatial equivalent to the temporal concept of reversibility and allows the application of thermodynamics to real systems, which are invariably nonequilibrium at large scales. Both local equilibrium and reversibility are examples of simplifying assumptions that allow us to treat complex situations. In making such assumptions, some accuracy in the answer may be lost. Knowing when and how to simplify a problem is an important scientific skill.
2.2.1 Fundamental thermodynamic variables
In the next two chapters we will be using a number of variables, or properties, to describe thermodynamic systems. Some of these will be quite familiar to you, others less so. Volume, pressure, energy, heat, work, entropy, and temperature are the most fundamental variables in thermodynamics. As all other thermodynamic variables are derived from them, it is worth our while to consider a few of these properties.
Читать дальше














