In Chapter 13, we will focus attention on the Critical Zone, which is the land surface from the top of vegetation to the bottom of circulating groundwater. It is so called because essentially all terrestrial life lives within it and ultimately all life, including marine life, depends on processes occurring within this zone. It is here that rock comes in contact with water and air, and primary minerals are replaced by new ones. These weathering reactions produce soil and release nutrients that make terrestrial life possible. Some fraction of these nutrients is carried to the oceans by streams and rivers and make marine life possible. Life is an integral part of the weathering and soil development process, as organic acids help to break down rock and movements of metals complexed by organic molecules contribute to the development of distinct soil horizons over time.
Weathering of silicate rocks is another important part of the carbon cycle and consequently influences climate on time scales of tens to hundreds of millions of years. This is because carbonic acid produced by dissolution of CO 2in water provides most of the acidity necessary to drive weathering reactions. The result is a solution enriched in calcium and bicarbonate, which is then carried to the oceans by streams and rivers to be precipitated as carbonate sediment, thus removing CO 2from the atmosphere until it is again released by metamorphism or volcanism to the atmosphere as CO 2. Over Earth's history, there has been a net transfer of CO 2from the atmosphere to sedimentary carbonate, keeping Earth's surface temperature within the habitable range even as the Sun has grown steadily brighter. We'll examine weathering reactions and their rates from the perspective of field studies. We'll find that lithology, climate and hydrology, topography, and the biota all exert important controls on weathering rates. We'll then turn our attention to the composition of streams and rivers and see how these same factors control the composition of streams and rivers. Finally, we look at the composition of saline lakes and see how the process of fractional crystallization leads to a great diversity of their compositions.
In Chapter 14, we follow the rivers to where they lead: the oceans. The oceans are salty and alkaline because, as Anton Lavoisier put it, they are “the rinsings of the Earth,” that is, they contain the weathering products of the land surface. Just six components, Na +, Mg 2+, Ca 2+, K +, Cl –, and 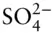 , make up 99.3% of the dissolved solids, and these are always present in the same proportions and in the same proportion to the total, the salinity , which is about 35 parts per thousand by weight on average. A final component,
, make up 99.3% of the dissolved solids, and these are always present in the same proportions and in the same proportion to the total, the salinity , which is about 35 parts per thousand by weight on average. A final component, 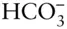 , brings the total to 99.7%.
, brings the total to 99.7%.
Ultimately, the concentrations of all components in seawater are controlled both by the rates at which they are added from sources and the rates at which they are removed by sinks . Rivers are the major source of most elements in seawater, but the atmosphere is the major source for dissolved gases as well as a few metals such as Al, Pb, and Th, which reach the ocean in wind-blown dust. Ridge crust hydrothermal activity is an important source of some elements, but it is also an important sink for others. Sediments are the major sink for most elements, and half the ocean floor is covered by the carbonate and siliceous shells of planktonic organisms. Evaporites are the major sink for Na +, K +, Cl –, and 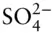 , but these form discontinuously through time. The last major evaporite deposit formed in the Mediterranean when tectonics closed the Strait of Gibraltar between 6 and 5.3 million years ago. The Mediterranean dried up nearly entirely, and the resulting drop in base level allowed rivers running into it, such as the Rhone and Nile, to cut channels 1000 m below their present levels, which subsequently filled with sediment when the Gibraltar connection reopened. The vast, thick beds of salt deposited beneath the Mediterranean during this time were enough to decrease global ocean salinity by 5%.
, but these form discontinuously through time. The last major evaporite deposit formed in the Mediterranean when tectonics closed the Strait of Gibraltar between 6 and 5.3 million years ago. The Mediterranean dried up nearly entirely, and the resulting drop in base level allowed rivers running into it, such as the Rhone and Nile, to cut channels 1000 m below their present levels, which subsequently filled with sediment when the Gibraltar connection reopened. The vast, thick beds of salt deposited beneath the Mediterranean during this time were enough to decrease global ocean salinity by 5%.
Biological processes exert an extremely important influence on ocean chemistry. Unlike the other major components, the concentration of 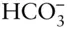 varies, mainly due to photosynthesis and respiration, although calcium carbonate precipitation and CO 2exchange with the atmosphere also contribute to variations. Photosynthesis is restricted by light availability to the upper hundred meters or so, while respiration occurs throughout the ocean. Temperature and salinity establish a strong density gradient in the ocean that has the effect of limiting exchange between this photic zone and the deep ocean. Once it is cooled at high latitudes and sinks into the deep ocean, water remains there on time scales of ∼1000 years. Sinking organic remains can fall through the water column and this density barrier and can be remineralized through respiration in the deep water. This transports dissolved CO 2from the surface to this deep water where it builds up, a phenomenon known as the biological pump . Consequently
varies, mainly due to photosynthesis and respiration, although calcium carbonate precipitation and CO 2exchange with the atmosphere also contribute to variations. Photosynthesis is restricted by light availability to the upper hundred meters or so, while respiration occurs throughout the ocean. Temperature and salinity establish a strong density gradient in the ocean that has the effect of limiting exchange between this photic zone and the deep ocean. Once it is cooled at high latitudes and sinks into the deep ocean, water remains there on time scales of ∼1000 years. Sinking organic remains can fall through the water column and this density barrier and can be remineralized through respiration in the deep water. This transports dissolved CO 2from the surface to this deep water where it builds up, a phenomenon known as the biological pump . Consequently 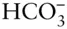 is present in higher concentration in deep water, which also results in a decrease in pH from ∼8.1 in the surface water to ∼7.6 in the deep water. Partly as a result of this variation in pH, the ocean becomes undersaturated with respect to calcium carbonate with depth so that carbonate shells formed in the surface water tend to dissolve of depth and do so completely below a depth at ∼4500 m. Falling carbonate shells also contribute to the biological pump, and as we noted above, this is also part of the long-term carbon cycle controlling climate. On much shorter time scales, changes in ocean circulation and biological productivity changed the efficiency of this biological pump between glacial and interglacial periods, resulting in a transfer of CO 2from the atmosphere to the deep ocean, very much amplifying the Milankovitch climate signal.
is present in higher concentration in deep water, which also results in a decrease in pH from ∼8.1 in the surface water to ∼7.6 in the deep water. Partly as a result of this variation in pH, the ocean becomes undersaturated with respect to calcium carbonate with depth so that carbonate shells formed in the surface water tend to dissolve of depth and do so completely below a depth at ∼4500 m. Falling carbonate shells also contribute to the biological pump, and as we noted above, this is also part of the long-term carbon cycle controlling climate. On much shorter time scales, changes in ocean circulation and biological productivity changed the efficiency of this biological pump between glacial and interglacial periods, resulting in a transfer of CO 2from the atmosphere to the deep ocean, very much amplifying the Milankovitch climate signal.
Unlike the major elements, concentrations of most minor and trace elements are quite variable in the oceans and much of this variation is due to biologic activity that imposes vertical concentration gradients, as these elements are taken up by phytoplankton in the surface water and released by respiration in the deep water. This includes not only nutrients such as P, Si, and Fe, but also nonutilized elements such as Ge because organisms take them up incidentally. A few elements, such as Al and Pb, show the opposite pattern: enrichment in the surface water and depletion in deep water because wind-deposited dust is the primary source of these elements and they are quickly scavenged onto particle surfaces after deposition.
In the final chapter we see how geochemistry can be used to address the needs of society, specifically, its need for mineral resources and environmental protection. The story of civilization is in some respects the story of increasingly sophisticated tools. The Stone Age ended when people learned to produce copper metal from copper sulfide ores around 7000 years ago. Copper tools were subsequently replaced by bronze ones and then by iron ones beginning around 3000 years ago. In a sense, we still live in the Copper and Iron Ages, however, as 21 million tons of copper ore and 2.5 billion tons of iron ore were mined globally in 2018. In the United States, about half the demand for metals is met by recycling, but modern society still need enormous amounts. Furthermore, modern technology requires a great variety of metals, many of which were unknown as recently as two centuries ago. At least 80 different elements are incorporated in smartphones or used in their production, including exotic ones like neodymium, europium, and tantalum. Two other exotic elements, cadmium and tellurium, are used to produce CdTe solar panels, which have the highest efficiency and can be produced in thinner films than other solar cells.
Читать дальше

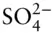 , make up 99.3% of the dissolved solids, and these are always present in the same proportions and in the same proportion to the total, the salinity , which is about 35 parts per thousand by weight on average. A final component,
, make up 99.3% of the dissolved solids, and these are always present in the same proportions and in the same proportion to the total, the salinity , which is about 35 parts per thousand by weight on average. A final component, 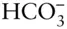 , brings the total to 99.7%.
, brings the total to 99.7%.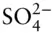 , but these form discontinuously through time. The last major evaporite deposit formed in the Mediterranean when tectonics closed the Strait of Gibraltar between 6 and 5.3 million years ago. The Mediterranean dried up nearly entirely, and the resulting drop in base level allowed rivers running into it, such as the Rhone and Nile, to cut channels 1000 m below their present levels, which subsequently filled with sediment when the Gibraltar connection reopened. The vast, thick beds of salt deposited beneath the Mediterranean during this time were enough to decrease global ocean salinity by 5%.
, but these form discontinuously through time. The last major evaporite deposit formed in the Mediterranean when tectonics closed the Strait of Gibraltar between 6 and 5.3 million years ago. The Mediterranean dried up nearly entirely, and the resulting drop in base level allowed rivers running into it, such as the Rhone and Nile, to cut channels 1000 m below their present levels, which subsequently filled with sediment when the Gibraltar connection reopened. The vast, thick beds of salt deposited beneath the Mediterranean during this time were enough to decrease global ocean salinity by 5%.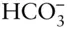 varies, mainly due to photosynthesis and respiration, although calcium carbonate precipitation and CO 2exchange with the atmosphere also contribute to variations. Photosynthesis is restricted by light availability to the upper hundred meters or so, while respiration occurs throughout the ocean. Temperature and salinity establish a strong density gradient in the ocean that has the effect of limiting exchange between this photic zone and the deep ocean. Once it is cooled at high latitudes and sinks into the deep ocean, water remains there on time scales of ∼1000 years. Sinking organic remains can fall through the water column and this density barrier and can be remineralized through respiration in the deep water. This transports dissolved CO 2from the surface to this deep water where it builds up, a phenomenon known as the biological pump . Consequently
varies, mainly due to photosynthesis and respiration, although calcium carbonate precipitation and CO 2exchange with the atmosphere also contribute to variations. Photosynthesis is restricted by light availability to the upper hundred meters or so, while respiration occurs throughout the ocean. Temperature and salinity establish a strong density gradient in the ocean that has the effect of limiting exchange between this photic zone and the deep ocean. Once it is cooled at high latitudes and sinks into the deep ocean, water remains there on time scales of ∼1000 years. Sinking organic remains can fall through the water column and this density barrier and can be remineralized through respiration in the deep water. This transports dissolved CO 2from the surface to this deep water where it builds up, a phenomenon known as the biological pump . Consequently 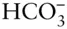 is present in higher concentration in deep water, which also results in a decrease in pH from ∼8.1 in the surface water to ∼7.6 in the deep water. Partly as a result of this variation in pH, the ocean becomes undersaturated with respect to calcium carbonate with depth so that carbonate shells formed in the surface water tend to dissolve of depth and do so completely below a depth at ∼4500 m. Falling carbonate shells also contribute to the biological pump, and as we noted above, this is also part of the long-term carbon cycle controlling climate. On much shorter time scales, changes in ocean circulation and biological productivity changed the efficiency of this biological pump between glacial and interglacial periods, resulting in a transfer of CO 2from the atmosphere to the deep ocean, very much amplifying the Milankovitch climate signal.
is present in higher concentration in deep water, which also results in a decrease in pH from ∼8.1 in the surface water to ∼7.6 in the deep water. Partly as a result of this variation in pH, the ocean becomes undersaturated with respect to calcium carbonate with depth so that carbonate shells formed in the surface water tend to dissolve of depth and do so completely below a depth at ∼4500 m. Falling carbonate shells also contribute to the biological pump, and as we noted above, this is also part of the long-term carbon cycle controlling climate. On much shorter time scales, changes in ocean circulation and biological productivity changed the efficiency of this biological pump between glacial and interglacial periods, resulting in a transfer of CO 2from the atmosphere to the deep ocean, very much amplifying the Milankovitch climate signal.










