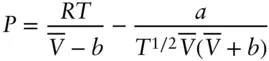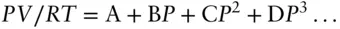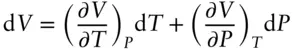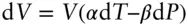(2.11) 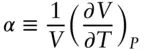
For an ideal gas, the coefficient of thermal expansion is simply the inverse of temperature.
The compressibility of a substance is defined in a similar manner as the fractional change in volume produced by a change in pressure at constant temperature:
(2.12) 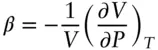
Geophysicists sometimes use the isothermal bulk modulus, K T, in place of compressibility. The isothermal bulk modulus is simply the inverse of compressibility: K T= 1/ β . Through a similar derivation to the one we have just done for the coefficient of thermal expansion, it can be shown that the compressibility of an ideal gas is β = 1/ P .
The ideal gas law can be derived from statistical physics (first principles), assuming the molecules occupy no volume and have no electrostatic interactions. Doing so, we find that R = N Ak, where k is Boltzmann's constant (1.381 × 10–23 J/K), N Ais the Avogadro number (the number of atoms in one mole of a substance), and k is a fundamental constant that relates the average molecular energy, e, of an ideal gas to its temperature (in Kelvins) as e = 3k T /2.
Since the assumptions just stated are ultimately invalid, it is not surprising that the ideal gas law is only an approximation for real gases; it applies best in the limit of high temperature and low pressure. Deviations are largest near the condensation point of the gas.
The compressibility factor is a measure of deviation from ideality and is defined as
(2.13) 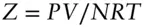
By definition, Z = 1 for an ideal gas.
2.3.2 Equations of state for real gases
2.3.2.1 Van der Waals equation
Factors we need to consider in constructing an equation of state for a real gas are the finite volume of molecules and the attractive and repulsive forces between molecules arising from electric charges. The van der Waals equation is probably the simplest equation of state that takes account of these factors. The van der Waals equation is:
(2.14) 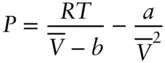
Here again we have converted volume from an extensive to an intensive property by dividing by N .
Let's examine the way in which the Van der Waals equation attempts to take account of finite molecular volume and forces between molecules. Considering first the forces between molecules, imagine two volume elements v 1and v 2. The attractive forces will be proportional to the number of molecules or the concentrations, c 1and c 2, in each. Therefore, attractive forces are proportional to 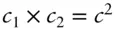 . Since c is the number of molecules per unit volume,
. Since c is the number of molecules per unit volume, 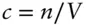 , we see that attractive forces are proportional to
, we see that attractive forces are proportional to 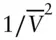 . The a term is a constant that depends on the nature and strength of the forces between molecules, and will therefore be different for each type of gas.
. The a term is a constant that depends on the nature and strength of the forces between molecules, and will therefore be different for each type of gas.
In the first term on the right, 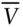 has been replaced by
has been replaced by 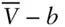 . b is the volume actually occupied by molecules, and the term
. b is the volume actually occupied by molecules, and the term 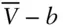 is the volume available for movement of the molecules. Since different gases have molecules of differing size, we can expect that the value of b will also depend on the nature of the gas. Table 2.1lists the values of a and b for a few common gases.
is the volume available for movement of the molecules. Since different gases have molecules of differing size, we can expect that the value of b will also depend on the nature of the gas. Table 2.1lists the values of a and b for a few common gases.
2.3.2.2 Other equations of state for gases
The Redlich-Kwong equation (1949) expresses the attractive forces as a more complex function:
(2.15) 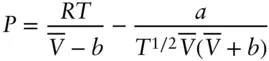
The Virial equation is much easier to handle algebraically than the van der Waals equation and has some theoretical basis in statistical mechanics:
(2.16) 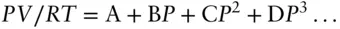
Table 2.1 Van der Waals constants for selected gases.
| Gas |
a liter-atm/mole 2 |
b liter/mole |
| Helium |
0.034 |
0.0237 |
| Argon |
1.345 |
0.0171 |
| Hydrogen |
0.244 |
0.0266 |
| Oxygen |
1.360 |
0.0318 |
| Nitrogen |
1.390 |
0.0391 |
| Carbon dioxide |
3.592 |
0.0399 |
| Water |
5.464 |
0.0305 |
| Benzene |
18.00 |
0.1154 |
A, B, C, .... are empirically determined (temperature-dependent) constants.
2.3.3 Equation of state for other substances
The compressibility and coefficient of thermal expansion parameters allow us to construct an equation of state for any substance. Such an equation relates the fundamental properties of the substance: its temperature, pressure, and volume. The partial differential of volume with respect to temperature and pressure is such an equation:
(2.17) 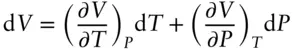
Substituting the coefficient of thermal expansion and compressibility for ∂V/∂T and ∂V/∂P respectively we have:
(2.18) 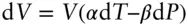
Thus, to write an equation of state for a substance, our task becomes to determine its compressibility and coefficient of thermal expansion. Once we know them, we can integrate eqn. 2.18to obtain the equation of state. These, however, will generally be complex functions of temperature and pressure, so the task is often not easy.
2.4 TEMPERATURE, ABSOLUTE ZERO, AND THE ZEROTH LAW OF THERMODYNAMICS
How do you define and measure temperature? We have discussed temperature with respect to the ideal gas law without defining it, though we all have an intuitive sense of what temperature is. We noted above that temperature of a gas is a measure of the average (kinetic) energy of its molecules. Another approach might be to use the ideal gas law to construct a thermometer and define a temperature scale. A convenient thermometer might be one based on the linear relationship between temperature and the volume of an ideal gas. Such a thermometer is illustrated in Figure 2.3. The equation describing the relationship between the volume of the gas in the thermometer and our temperature, τ, is:
Читать дальше

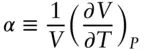
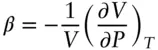
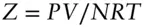
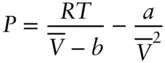
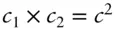 . Since c is the number of molecules per unit volume,
. Since c is the number of molecules per unit volume, 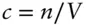 , we see that attractive forces are proportional to
, we see that attractive forces are proportional to 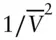 . The a term is a constant that depends on the nature and strength of the forces between molecules, and will therefore be different for each type of gas.
. The a term is a constant that depends on the nature and strength of the forces between molecules, and will therefore be different for each type of gas.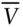 has been replaced by
has been replaced by 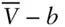 . b is the volume actually occupied by molecules, and the term
. b is the volume actually occupied by molecules, and the term 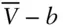 is the volume available for movement of the molecules. Since different gases have molecules of differing size, we can expect that the value of b will also depend on the nature of the gas. Table 2.1lists the values of a and b for a few common gases.
is the volume available for movement of the molecules. Since different gases have molecules of differing size, we can expect that the value of b will also depend on the nature of the gas. Table 2.1lists the values of a and b for a few common gases.