1 ...7 8 9 11 12 13 ...39 In addition to the membrane lipids that are derivatives of glycerol, animal cells contain additional lipids and phospholipids. These have the amino alcohol sphingosineas a base and are referred to as sphingolipids. The N ‐acyl fatty acid derivatives of sphingosine are termed ceramides. Sphingomyelin, one of the most important of the sphingolipids, has a structure analogous to that of phosphatidylcholine ( Figure 2.3). It is very common in the myelin sheathsfound around the axons of neurons.
If the sphingomyelin head group is substituted with a sugar residue (e.g. galactose or glucose), a cerebrosideresults. These membrane lipids are missing the phosphate residue and are therefore uncharged. Cerebrosides are common in the brain, where they are oriented toward the cell exterior. Gangliosidesare sphingolipids with an especially complex structure. They contain oligosaccharides and at least one sialic acidunit ( Figure 2.4). In the brain, 6% of lipids are present in the form of gangliosides. Sphingolipid storage diseases (e.g. Tay–Sachs disease), which result in early neurological deterioration, are of great medical importance.
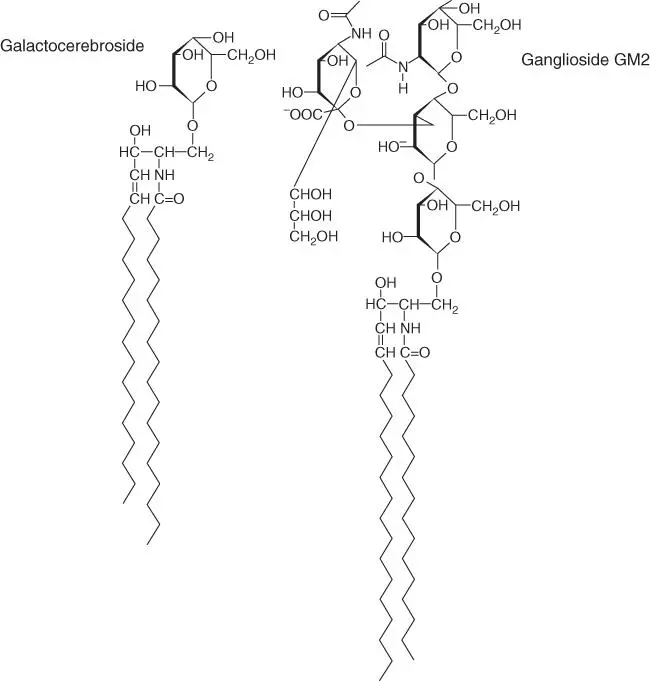
Figure 2.4 Chemical structure of cerebrosides (glycolipids). (a) Galactocerebroside and (b) ganglioside (GM2).
Phospholipids are cleaved by different phospholipases. Phospholipase A 2cleaves the central fatty acid at C2 of glycerol residues. The resulting lysophospholipid can lyse cell membranes; interestingly, many snake venoms contain high dosages of phospholipase A 2. Phospholipase A 1hydrolyzes the fatty acid at C1 of glycerol, while phospholipase Copens the phosphate ester bonds with glycerol.
A pharmacologically important lipid class, the eicosanoids, is only mentioned briefly here. To summarize, this class includes prostaglandins, thromboxanes, and leukotrienes. These play many roles and act as paracrine mediators (e.g. in pain, fever, inflammation, blood pressure, and blood coagulation). Phospholipase A 2releases arachidonic acidfrom phosphatidylcholine, which contains the fourfold unsaturated arachidonic acid in its C2 position. Arachidonic acid is converted into prostaglandin (e.g. example by cyclooxygenase). This enzyme is an important target for many drugs (the so‐called nonsteroidal anti‐inflammatory drug s [ NSAID s]), among which aspirin (acetylsalicylic acid) is the most famous. Inflammation can also be effectively suppressed by inhibiting the expression of phospholipase A 2by corticoids (e.g. cortisone medications).
Triacylglycerides, not phospholipids, are present in the storage tissueof plants and animals. These are broken down by lipases.
The steroid cholesterol( Figure 2.5) is an important and common building block of animal membranes (it is missing in the membranes of bacteria, fungi, and plants). It is stored in the membrane, parallel to the phospholipids ( Figure 2.2), with its polar hydroxyl group oriented toward the cell exterior. Cholesterol is a stiff molecule that stabilizes biological membranes and lowers their fluidity and permeability. In biological membranes, local assemblies of membrane proteins usually rich in cholesterol, known as rafts, have been found. Cholesterol is transported as cholesteryl ester, such as cholesterol‐3‐stearatein lipoproteins (see Chapter 5.4).
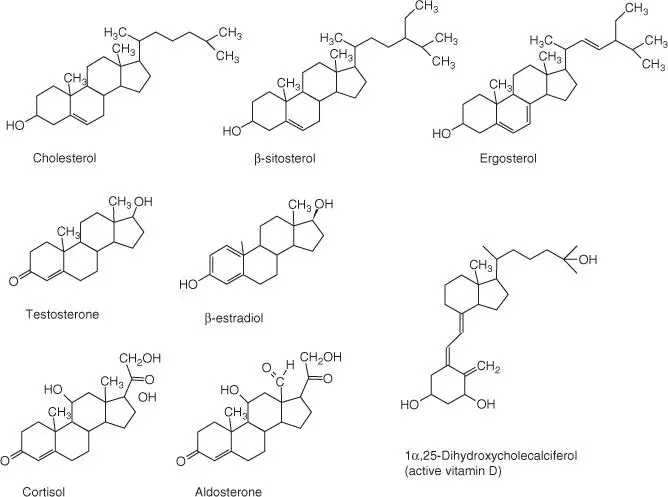
Figure 2.5 Cholesterol and related sterols. Cholesterol; β ‐sitosterol replaces cholesterol in plants; ergosterol is present in the membranes of fungi; testosterone; β ‐estradiol; cortisol; aldosterone; active vitamin D.
Cholesterolcan be synthesized in the body; the biggest portion, however, is obtained from food. It is important not only to build up membranes but also as a precursor for the synthesis of important hormones and vitamins ( Figure 2.5):
Glucocorticoids. For example, cortisol (from the adrenal gland) influences the metabolism of carbohydrates, proteins, and lipids; cortisol inhibits phospholipase A2, induces several genes such as the transcription factor NF‐κB, and thus suppresses inflammation processes.
Mineralocorticoids. For example, aldosterone (from the adrenal gland) regulates the secretion of salt and water through the kidneys.
Sexual hormones. Androgens (testosterone, formed in the testicles) and estrogens (β‐estradiol, formed in the ovaries) are important male and female sexual hormones. They bind intracellular receptors that, as transcription factors, control the expression of sex‐dependent genes (see Section 4.2).
Vitamin D. Vitamin D increases the calcium concentration in the blood and assists in the formation of bones and teeth. Vitamin D deficiency is known as rickets in children and osteomalacia in adults.
2.3 Structure and Function of Proteins
Proteins represent the most important tools of the cell (Table 2.2). They catalyze chemical reactions, transport metabolites through membranes, recognize other molecules, and can regulate gene activity. If we consider genes as the legislative branch, proteins then function as the executive branch (i.e. as the executing organs). Proteins are built according to the same principles in both prokaryotes and eukaryotes.
Twenty amino acids serve as building blocks for peptides and proteins, linked to one another by peptide bonds( Figure 2.6). Polypeptides, therefore, are polymers made from amino acids. Polypeptides are polar molecules, possessing a NH 2group ( amino‐ or N‐terminal) on one end and a COOH group ( carboxyl‐ or C‐ terminal) on the other. The diverse tasks and functions of proteins result from different arrangements (sequences) of amino acids.
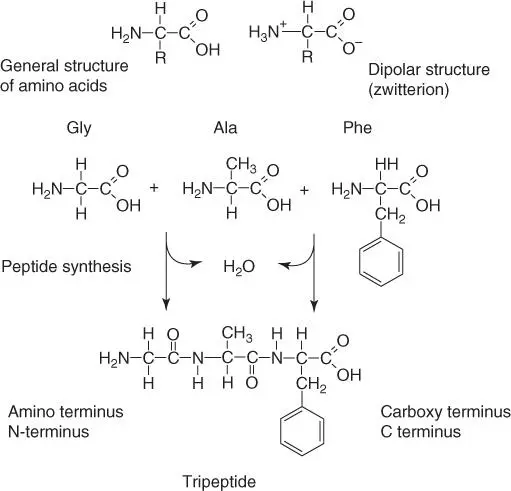
Figure 2.6 General structure of amino acids and peptides.
The 20 amino acids differ in their side chains ( Figure 2.7). The functional groups of the side chains, which protrude from the α ‐C atom, dictate the conformation and later functionality of the protein by molecular recognition or biocatalysis. Amino acids exist in two optical isomers: the D‐ and L‐forms. Polypeptides are composed exclusively of L‐amino acids. D‐Amino acidscan be found in bacterial cell walls and in many antibiotics(gramicidin, valinomycin). Since proteases can only cleave peptides composed of L‐amino acids, the incorporation of D‐amino acids results in a certain protection from untimely degradation.
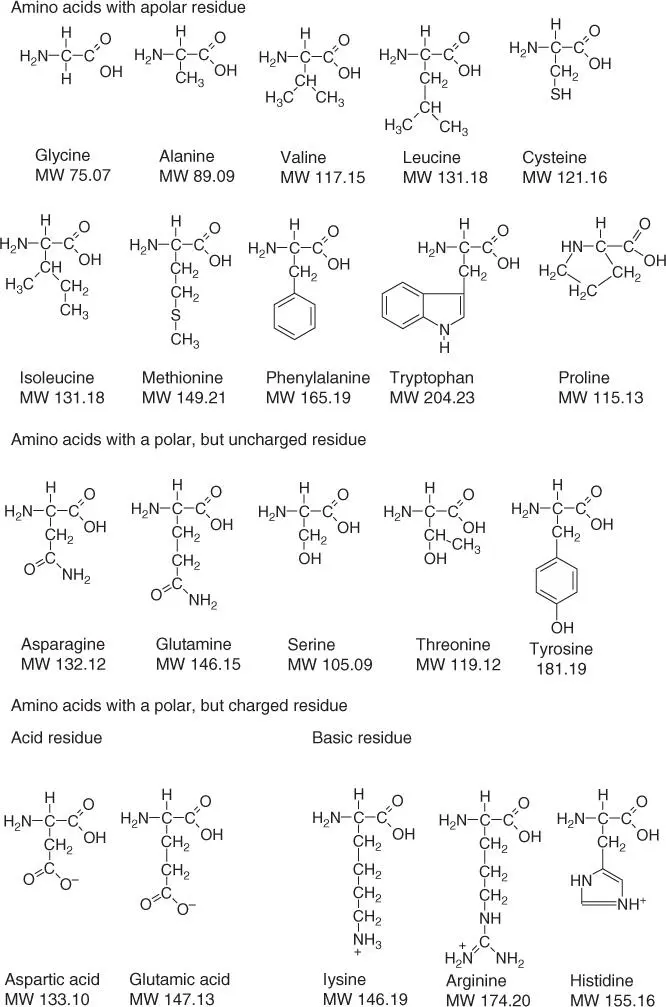
Figure 2.7 Structures of proteinogenic amino acids. (Cysteine muss zu den amino acids with apolar residues.)
Читать дальше







![Andrew Radford - Linguistics An Introduction [Second Edition]](/books/397851/andrew-radford-linguistics-an-introduction-second-thumb.webp)








