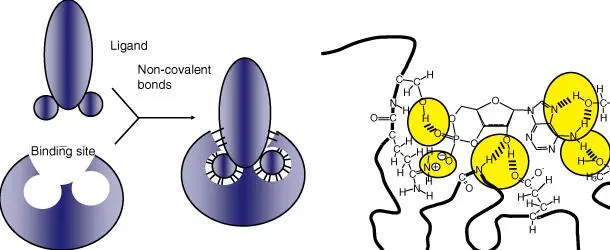
Figure 2.15 Structure of binding sites within proteins. (a) Schematic illustration of the significance of noncovalent bonds in the lock‐and‐key principle. (b) cAMP is locked into a binding site via ionic and hydrogen bonds.
Interactions that occur between antigensand antibodies(see Chapter 28), between ligandsand hormonereceptors, and between enzymesand their substratesare particularly intimate and selective. The topic of protein–protein interactions is discussed further in Chapter 23.
Most of the cellular building blocks are inert molecules that are not prone to react chemically. Significant activation energyhas to be overcome in order to start an energy‐consuming chemical reaction. In the laboratory, this can be achieved by heating and adding acids or bases. In biological systems, evolution has developed enzymes as biological catalyststhat are able to catalyze all necessary reactions without higher temperatures being necessary. Enzymes do not change the reaction equilibrium, but usually alter the reaction rate. Enzymes contain an active center in which a substrate is bound. After the enzyme has catalyzed a reaction, the product is released, but the enzyme remains unchanged and is ready for a new reaction. Noncovalent interactions (hydrogen bonds, ionic bonds) and transient covalent bonds between protein and substrate play a key role during the binding and catalysis. Detailed elucidation of such interactions at the atomic scale is the task of biophysics and biochemistry. This research is also important for biotechnology in relation to the synthesis of new enzyme inhibitors or enzyme modulators.
Enzymes show high substrate specificity. It is believed that for almost every biosynthetic step that happens in the cell, a specific enzyme is also present. This does not rule out that enzymes that catalyze chemically similar reactions can be derived from a common original enzyme. Such enzymes belong to a common protein family. Most enzymes have particular pHand temperature optima. Enzymes are divided into different classes according to the processes catalyzed (Table 2.5). Coenzymesor inorganic ionsoften take part in the catalysis itself. Many coenzymes must be ingested in the forms of vitamins (Table 2.6) because the human body cannot synthesize them themselves. Biochemists and biotechnologists are interested in the elucidation of the enzymatic reaction mechanisms because hints for new catalysts for organic synthesis can be obtained. Apart from this, scientists are attempting to create new biological catalysts through the production of artificial enzymes through genetic engineering of existing enzymes.
Table 2.5 Important classes of enzymes.
| Enzyme |
Reaction catalyzed |
| Hydrolases |
Catalyze hydrolytic cleavage (amylase, lipase, glucosidase, esterase) |
| Nucleases |
Hydrolyze nucleic acids (DNase, RNase) |
| Proteases |
Cleave peptides (pepsin, trypsin, chymotrypsin) |
| Isomerases |
Catalyze the rearrangement of bonds within a molecule |
| Synthases |
General name for an enzyme that catalyzes condensation reactions in anabolic processes |
| Polymerases |
Catalyze the formation of RNA and DNA |
| Kinases |
Transfer phosphate residues; the protein kinases (PKA, PKC) are particularly important |
| Phosphatases |
Remove phosphate residues from a molecule |
| ATPases |
Hydrolyze ATP (e.g. H +‐ATPase, Na +, K +‐ATPase, Ca 2+‐ATPase); motor proteins, such as myosin |
| GTPases |
Hydrolyze GTP; many GTP‐binding proteins work as GTPases |
| Oxidoreductases |
Enzymes that catalyze redox reactions, in which one molecule is reduced and another is oxidized; they are grouped into oxidases, reductases, and dehydrogenases |
Table 2.6 Many vitamins serve as essential coenzymes for enzyme reactions.
| Vitamin |
Coenzyme |
Enzyme reactions that require the coenzyme |
| Thiamine (vitamin B 1) |
Thiamine pyrophosphate |
Activation and transfer of aldehydes |
| Pyridoxine (vitamin B 6) |
Pyridoxal phosphate |
Transaminases and decarboxylases |
| Biotin (vitamin B 7) |
Biotin |
Activation and transfer of CO 2 |
| Riboflavin (vitamin B 2) |
FADH |
Oxidations–reductions |
| Niacin (vitamin B 3) |
NADH, NADPH |
Oxidations–reductions |
| Pantothenic acid (vitamin B 5) |
Coenzyme A |
Activation and transfer of acyl groups |
| Lipoic acid |
Lipoamide |
Activation of acyl groups; oxidation–reductions |
| Folic acid (vitamin B 9) |
Tetrahydrofolate |
Activation and transfer of single‐carbon groups |
| Vitamin B 12 |
Cobalamin |
Isomerization and methyl group transfer |
In addition to a catalytic center, many enzymes (especially those composed of several subunits) also have a regulatory centerwhere allosteric ligandsbind. For example, the second messenger cAMP binds to the tetrameric protein kinase A complex; after binding both regulatory protein subunits dissociate from both catalytic subunits, which results in their activation ( Figure 3.9). Enzymes can be inhibited by inhibitors. We distinguish between reversible, irreversible, competitive, and noncompetitive inhibitors.
A further important way to regulate the activity of enzymes or regulatory proteins is that of reversible conformational change. This is achieved by phosphorylation/dephosphorylation with the help of protein kinasesor phosphatases, respectively. Most of the protein kinases utilize adenosine triphosphate (ATP); other molecular switcheswork through the binding of guanosine triphosphate ( GTP )and guanosine diphosphate ( GDP )( Figure 2.16, Table 2.7). A reversible reduction of disulfide bridges(e.g. through thioredoxin) plays an important role during the regulation of light‐dependent chloroplast enzymes. Biochemists and cell biologists are working extensively to define all cellular proteins that are regulated through phosphorylation and GTP/GDP to gain a better understanding of regulation processes and regulatory pathways or networks inside the cell (see Section 3.1.1.3).
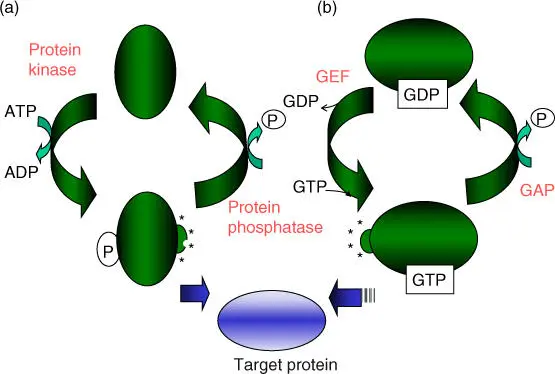
Figure 2.16 Reversible activation and inactivation of enzymes and regulatory proteins. (a) Phosphorylation/dephosphorylation. (b) Binding of GTP/GDP. GEF, guanine nucleotide exchange factor; GAP, GTPase‐activating protein.
Table 2.7 Nomenclature of DNA and RNA building blocks.
| Base |
Nucleotide (abbreviation) |
Nucleotide (number of phosphate groups) |
|
|
RNA |
DNA |
|
|
1 |
2 |
3 |
1 |
2 |
3 |
| Adenine |
Adenosine (A) |
AMP |
ADP |
ATP |
dAMP |
dADP |
dATP |
| Guanine |
Guanosine (G) |
GMP |
GDP |
GTP |
dGMP |
dGDP |
dGTP |
| Cytosine |
Cytidine (C) |
CMP |
CDP |
CTP |
dCMP |
dCDP |
dCTP |
| Thymine |
Thymidine (T) |
|
|
|
dTMP |
dTDP |
dTTP |
| Uracil |
Uridine (U) |
UMP |
UDP |
UTP |
|
|
|
AMP, adenosine monophosphate; ADP, adenosine diphosphate; ATP, adenosine triphosphate; d, deoxy.
Читать дальше





![Andrew Radford - Linguistics An Introduction [Second Edition]](/books/397851/andrew-radford-linguistics-an-introduction-second-thumb.webp)








