
Figure 3.1 Mobility of phospholipids in a biomembrane. Three types of movement are possible: rotation (spin), lateral diffusion, and flip‐flop, which occurs rarely. A flip‐flop can be brought about with the enzyme flippase.
Under cellular conditions, biomembranestend not to lie flat like a carpet, but assume a spherical shape ( Figure 3.2a). Should holes and ruptures in the cytoplasmic membrane occur, they are only transient and immediately resealed. These remarkable self‐organization and formation of supramolecular structures were prerequisites for the emergence of cells – and thus of life itself. Membranes can easily invert to form vesiclesthat, in turn, can merge with other membranes. When a vesicle is pinched off from a biomembrane, this is called exocytosis. When a vesicle is absorbed by a compartment membrane, it is called endocytosis.
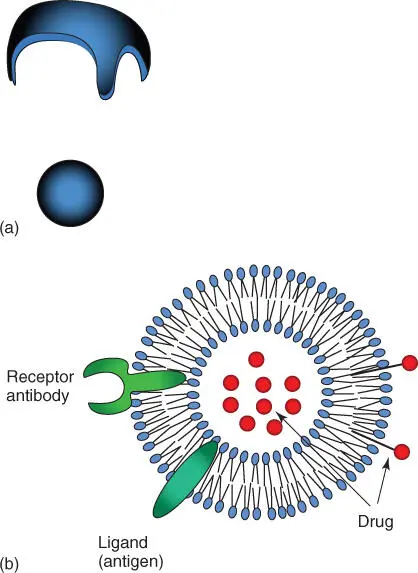
Figure 3.2 Vesicle and liposome formation. (a) In an aqueous environment, lipid bilayers spontaneously form spherical vesicles, which makes them energetically favorable. (b) Schematic view of a liposome. Receptors, antibodies, and ligands may be integrated into the outside, which enables the liposome to recognize its target. Active compounds may be stored inside the liposome or bound outside of the membrane using nanoparticles or carrier molecules.
Small closed vesicles consisting of synthetic phospholipids are also called liposomes( Figure 3.2b). These play an important role in medicine and biotechnology, as they serve as vehicles for pharmaceutical compounds. They can be loaded with aggressive toxins. Researchers are trying to modify liposomes so that they can direct them to their targets via receptors or antibodies that are embedded in the liposomal membrane (see Chapter 26). This could prevent chemotherapeutics, such as those used in cancer therapy, from attacking and damaging healthy cells.
Cellular membranes have an asymmetric structure. Their building blocks on the inside of the cell differ from those on the outside ( Figure 3.3). Due to the presence of negatively charged phosphatidylserine, the inside of a membrane is negatively charged. Biomembranes owe their specificity to the integration of certain membrane proteins and lipids. In the ER, new membrane sections are synthesized, allowing for their asymmetric structure. The enzyme flippasehas an additional role to play in this context – facilitating a change of orientation in individual phospholipids ( Figure 3.1).
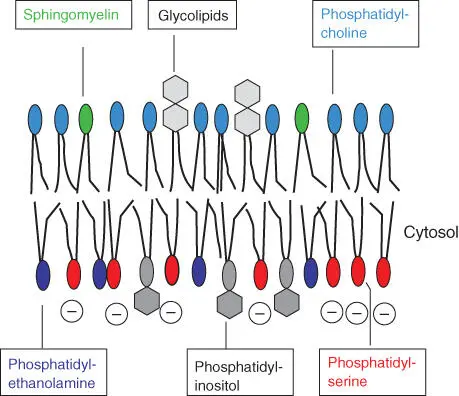
Figure 3.3 Asymmetric structure of biomembranes.
3.1.1.1 Membrane Permeability
Biomembranes serve primarily as permeability barriers. The lipophilic inside of the membrane is an effective barrier against the diffusion of polar and charged substances, while membrane proteinsenable the controlled import and export of ions and metabolites. The effectiveness of the membrane as a permeability barrier becomes apparent when looking at the difference in ion concentrations inside and outside a cell (Table 3.1). The differences in ion concentration may be as large as several powers of 10.
Table 3.1 Ion concentrations inside mammalian cells and in the extracellular space.
| Ion |
Intracellular concentration |
Extracellular concentration |
| Cations |
| Na + |
5–15 mM |
145 mM |
| K + |
140 mM |
5 mM |
| Mg ++ |
0.5 mM a) |
1–2 mM |
| Ca ++ |
100 nM a) |
1–2 mM |
| H + |
10 −7.2M(=pH 7.2) |
10 −7.4M(=pH 7.4) |
| Anions |
| Cl − |
5–15 mM |
110 mM |
a)Ca ++and Mg ++also occur bound to proteins within the cell (1–2 mM or 20 mM, respectively).
Figure 3.4shows a schematic view of the barrier function, using the example of an artificial lipid bilayer. Given sufficient time, any substance will diffuse through a membrane. The diffusion rate, however, varies considerably, depending on size, charge, and lipophilic properties of a molecule. The smaller and more hydrophobic a molecule is, the faster it will diffuse across a cell membrane. The following rules apply:
Smaller nonpolar molecules such as O2, CO2, and N2 are lipid soluble, diffusing rapidly through biomembranes. This is also true for lipophilic organic molecules such as benzene or chloroform. Many therapeutics are strongly lipophilic and can thus diffuse freely into the body.
Small uncharged polar molecules are slightly slower to diffuse through the membrane. This category includes molecules such as H2O, ethanol, urea, or glycerol.
The biomembrane forms an effective barrier to larger charged molecules (sugar, amino acids, and nucleotides).
Small charged inorganic ions such as Na+, K+, Ca2+, or Cl− are unable to permeate the lipid bilayer through free diffusion.
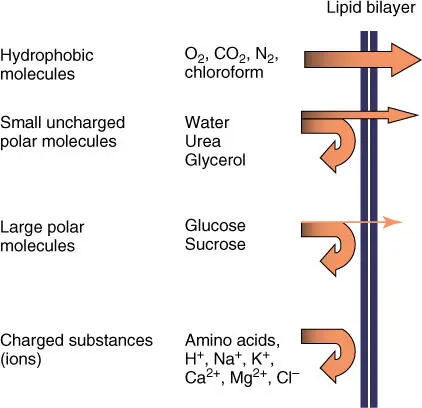
Figure 3.4 Permeability of artificial lipid membranes for biologically relevant substances.
Membrane permeability can be modified by certain agents. For example, bacteria are vulnerable to antimicrobial peptides (AMPs) and certain antibiotics, such as the peptide antibiotics tyrothricin, polymyxin B, gramicidin, and valinomycin or the polyene antibiotic amphotericin B. These substances act on the biomembrane and disrupt the ion balance specifically or nonspecifically; some are ionophores. Many plants produce saponins, which unselectively disturb membrane permeability. Furthermore, the effect of some inhaled anesthetics can be interpreted as a disturbance of the biomembrane and of the ion channels.
3.1.1.2 Transport Processes Across Biomembranes
The properties of artificial lipid bilayers ( Figure 3.4) also apply to biomembranes. Water and other small nonpolar molecules enter the cell by free diffusion. While cells possess additional specific water absorption mechanisms (aquaporins), they also need to take up polar and charged nutrients and to release waste products. Polar charged components include inorganic ions, sugars, amino acids, organic acids, nucleotides, and various other metabolites. As normal diffusion through the membrane would be too slow, the cell uses specific membrane proteins to speed up the process ( Figure 3.5):
Ion channels or ion pumps for inorganic ions, above all Na+ channels, K+ channels, Ca2+ channels, and Cl− channels. More than 100 types of ion channels have been described. Ion channels exhibit ion selectivity and possess a sort of selectivity filter. Their activity can be modulated by voltage changes (voltage‐gated channel), by binding of ligands (ligand‐gated channel), and by mechanical stress (mechanically gated channel).
Transporters or carriers for organic molecules.
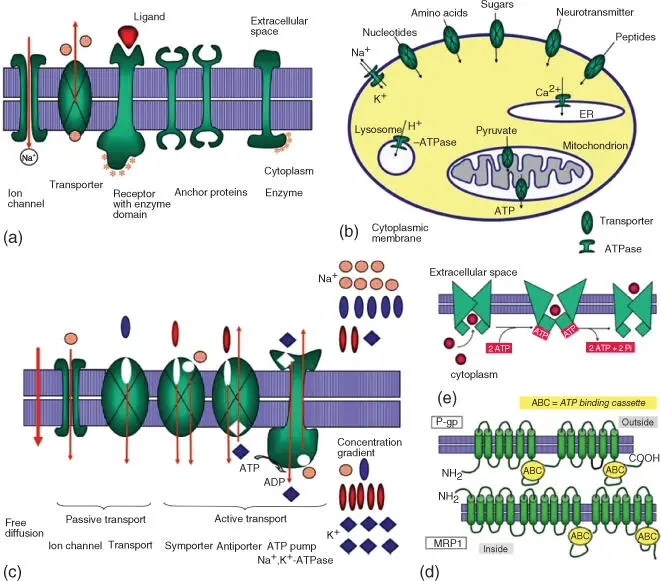
Figure 3.5 Important membrane proteins and transport processes. (a) Schematic view of ion channels, transporters, receptors, enzymes, and protein anchors. (b) Comparison of simple diffusion and active and passive transport. (c) Examples of transporters and ion pumps in an animal cell. (d) Mechanism of ABC transporters. (e) Structures of the MDR proteins P‐gp and MRP1.
Читать дальше








![Andrew Radford - Linguistics An Introduction [Second Edition]](/books/397851/andrew-radford-linguistics-an-introduction-second-thumb.webp)








