1 ...8 9 10 12 13 14 ...39 The proteinogenic amino acids can be divided into different groups according to their functional groups and residues ( Figure 2.7and Table 2.4):
Amino acids with apolar, lipophilic residues.
Amino acids with polar but uncharged residues (i.e. with hydroxyl or amide groups).
Amino acids with acid groups that are negatively charged.
Amino acids with basic groups that are positively charged.
Table 2.4 Compilation and grouping of the proteinogenic amino acids: two types of abbreviations are recognized internationally, which either consist of one or three letters; the codons that represent the amino acids in the genetic code are also given.
| Classification |
Symbols |
Codons |
| Neutral and nonpolar amino acids |
| Glycine |
Gly; G |
GGA GGC GGG GGU |
| Alanine |
Ala; A |
GCA GCC GCG GCU |
| Valine |
Val; V |
GUA GUC GUG GUU |
| Leucine |
Leu; L |
UUA UUG CUA CUC CUG CUU |
| Isoleucine |
Ile; I |
AUA AUC AUU |
| Tryptophan |
Trp; W |
UGG |
| Phenylalanine |
Phe; F |
UUC UUU |
| Methionine |
Met; M |
AUG |
| Cysteine |
Cys; C |
UGC UGU |
| Proline |
Pro; P |
CCU CCC CCA CCG |
| Neutral and polar amino acids |
| Serine |
Ser; S |
AGC AGU UCA UCC UCG UCU |
| Threonine |
Thr; T |
ACA ACC ACG ACU |
| Tyrosine |
Tyr; Y |
UAC UAU |
| Asparagine |
Asn; N |
AAC AAU |
| Glutamine |
Gln; Q |
CAA CAG |
| Basic amino acids |
| Lysine |
Lys; K |
AAA AAG |
| Arginine |
Arg; R |
AGA AGG CGA CGC CGG CGU |
| Histidine |
His; H |
CAC CAU |
| Acidic amino acids |
| Aspartate |
Asp; D |
GAC GAU |
| Glutamate |
Glu; E |
GAA GAG |
The human body is capable of synthesizing some amino acids; others must be obtained through nutrition (essential amino acids). The amino acids phenylalanine, tryptophan, lysine, methionine, valine, leucine, isoleucine, histidine, and threonine belong to the essential amino acids.
Proteins often undergo posttranslational modification, by transferring oligosaccharide residues to asparagine ( N‐glycosidic) or serine residues ( O‐glycosidic) (see Section 5.4). Glycoproteinsare found on the outside of the cell, in cell walls, and in the extracellular matrix, especially in connective tissue. Glycosylation is important for the biological activity and antigenic properties.
While the peptide bond itself is inflexible, the substituents at the α ‐C atom of an amino acid can rotate freely. As a result, a polypeptide chain can engage in a number of spatial structures ( conformations). Under aqueous conditions found in the cell, the polypeptide chains are not present in a linear form, but form spontaneous secondaryand tertiary structures, which are energetically more favorable. These structures rely on many noncovalent bonds and forces; those that are important include the following:
Hydrogen bonds (bond strength of 4 kJ mol–1 under aqueous conditions).
Ionic bonds (electrostatic attraction) (bond strength of 12.5 kJ mol–1).
van der Waals forces (bond strength of 0.5 kJ mol–1).
Hydrophobic attractions.
Figure 2.8summarizes the most common hydrogen bonds present in a cell. Electronegative atoms, such as oxygen and nitrogen, try to withdraw electrons from neighboring atoms such as hydrogen. This results in oxygen and nitrogen having a slight negative charge, while hydrogen is slightly positively charged. Positive and negative charges attract one another. The resulting attractions are known either as hydrogen bonds or as hydrogen bridges. The ability to form hydrogen bonds is especially present in water molecules (the hydrogens are positive; the oxygen atom is negatively charged), and water is therefore considered as the universal solvent of the cell. Biomolecules with polar groups easily take up water molecules (they are water soluble), while nonpolar residues repel water ( hydrophobic) and group together with other apolar molecules (which are fat soluble). Figure 2.9illustrates the importance of noncovalentand covalentbonds for the formation of protein folds. Through the formation of disulfide bridgesbetween two cysteine residues, the conformation of a protein can also be covalently influenced ( Figure 2.9).
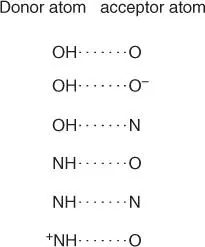
Figure 2.8 Important hydrogen bonds in biomolecules.
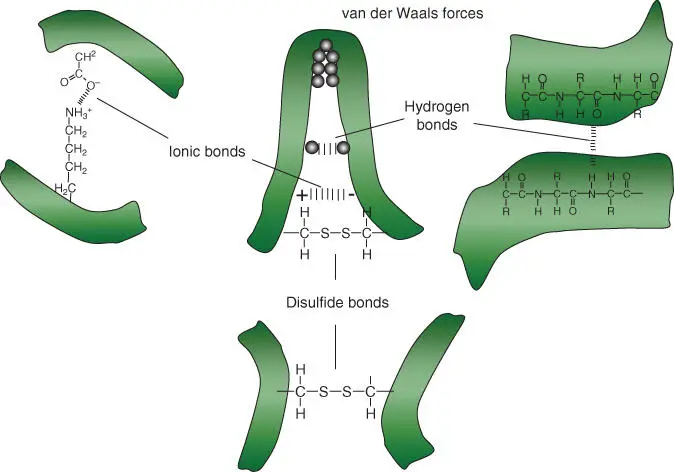
Figure 2.9 Noncovalent bonds and disulfide bridges lead to a spatial folding and stabilization of a peptide. Bond types: hydrogen bonds, ionic bonds, van der Waals forces, and disulfide bridges.
In comparison with covalent bonds(bond strength of 348–469 kJ mol –1), noncovalent bondsare 5–100 times weaker. When many noncovalent bonds are present, they simultaneously can work cooperatively, leading to the formation of stable and thermodynamically favored structure elements in polypeptides. Hydrophobic amino acid residues cluster together in order to lock water out. In polypeptides this can lead to a globular tertiary structure, while the hydrophobic residues are oriented toward the inside, and the polar and charged residues are oriented toward the outside ( Figure 2.10). Under aqueous conditions, proteins usually fold spontaneously into a stable conformation in which the free energy is at the lowest.
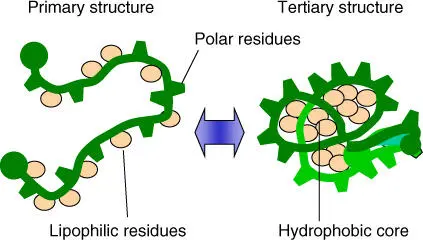
Figure 2.10 Folding of peptide chains under aqueous conditions leads to a compact globular conformation with a hydrophobic core.
However, the conformation of proteins can easily change if they come into contact with other proteins or contents of the cell. Other examples of protein modifications are phosphorylation(of hydroxyl groups of tyrosine, serine, and threonine) or dephosphorylationthat leads to a change in conformation. It is experimentally simple to alter the conformation of a protein using detergents or urea. For example, when globular proteins are dissolved in a 4 M ureasolution, the polypeptide chain unfolds (i.e. the protein is denatured). If the urea is removed, the polypeptide chain refolds into the previous conformation ( renaturing).
Even though each protein has an individual conformation, when the structures of many proteins are compared, two folding patterns that regularly appear are recognized. These structural elements are:
α‐Helix structures.
β‐Pleated sheet structures.
α‐Helix structuresand β ‐pleated sheet structures arise from hydrogen bonds between the NH and CO groups in the backbone of the polypeptide chain. Functional groups on the side chains do not take part in these structural elements. Figure 2.11describes the structure of helices and pleated sheets more precisely. Other structures include loops and random coils.
Читать дальше






![Andrew Radford - Linguistics An Introduction [Second Edition]](/books/397851/andrew-radford-linguistics-an-introduction-second-thumb.webp)








