1 ...6 7 8 10 11 12 ...39 Sugar molecules can be easily activated through esterification with an acid, one important example being esterification with phosphoric acid. Sugar phosphates are important in glycolysis.
The most important polysaccharide in animal cells is glycogen, which is stored as an energy source in liver and muscle. Glycogen can be quickly converted into glucose‐1‐phosphate and then channeled into glycolysis. Glycogen is a branched polysaccharide formed from glucose molecules linked by α ‐(1→4)‐glycosidic bonds or α ‐(1→6)‐glycosidic bonds ( Figure 2.1d). This results in many free ends on which the enzyme glycogen phosphorylase can begin degradation simultaneously.
Starchor amylose( Figure 2.1d) consists of glucose residues linked by α ‐(1→4)‐glycosidic bonds. In amylopectin, additional glucose residues linked by α ‐(1→6)‐glycosidic bonds are built in. Amylopectin, therefore, has a similar structure to glycogen but is less strongly branched. Starch is formed by photosynthesisin plant cells, where it is stored in amyloplasts. Starch can be broken down easily by animals and is therefore an important part of human nutrition.
Glucose is also used as a building block for cellulose( Figure 2.1d), which is necessary for formation of the plant cell wall. Cellulose is an unbranched polymer made from glucose molecules linked by β ‐(1→4)‐glycosidic bonds. Cellulose cannot be broken down in the human digestive tract. Conversely, the rumen (first stomach) of ruminants (animals that chew the cud) contains microorganisms that produce cellulase– an enzyme that makes it possible for cows, for example, to use cellulose as a nutrient. Additional polymers present in the plant cell wall include polysaccharides, so‐called glycans made up of cellulose fibers linked together in a diagonal fashion, pectin(basic unit: galacturonic acid), and lignin(made from the coumaroyl, coniferoyl, and sinapoyl alcohols). Using cellulases, it is possible to digest the cell walls of plant cells. Cells without cell walls are called protoplasts. They are important in plant biotechnology because they are easily transformable by genetic engineering (see Chapter 30). In many plant species it is possible to regenerate intact plant cells from protoplasts. Cell walls of fungi and the exoskeletons of insects are composed of chitin, which has N ‐acetylglucosamine as a building block in β ‐(1→4)‐glycosidic bonds.
Further important polysaccharides are found in animals. Hyaluronic acidis made up of many disaccharide building blocks, which themselves consist of glucuronic acid and N ‐acetylglucosamine. Hyaluronic acid has a very high viscosity and is therefore found in synovial fluid in the joints and in the vitreous humor in the eye. Furthermore, polysaccharides made from disaccharides consisting of sulfated glucuronic acidand N ‐acetylglucosamine or N ‐acetylgalactosamine units, respectively, are found in the connective tissues. Examples include chondroitin‐4‐sulfate, chondroitin‐6‐sulfate, dermatan sulfate, and keratin sulfate. Heparin, involved in the control of blood coagulation, also falls into this structural group. These polysaccharides are charged molecules under physiological conditions and can therefore interact with cellular macromolecules, such as proteins and nucleic acid by form hydrogen bridges and ion bonds.
2.2 Structure of Membrane Lipids
Biological membranes consist of a lipid bilayer( Figure 2.2). They are formed from phospholipids, glycolipids, and sterols(e.g. in animal membranes, cholesterol), which have lipophilic(fat loving, water repelling) and hydrophilic(water loving, fat repelling) structural elements. The lipid composition differs between cell types and compartments. Furthermore, biomembranes carry a diversity of membrane proteins (see Chapter 3). Biomembranes generate a diffusion barrier and enclose all cells and in eukaryotes enclose all internal organelles (mitochondria, plastids) and compartments (see Chapter 3).
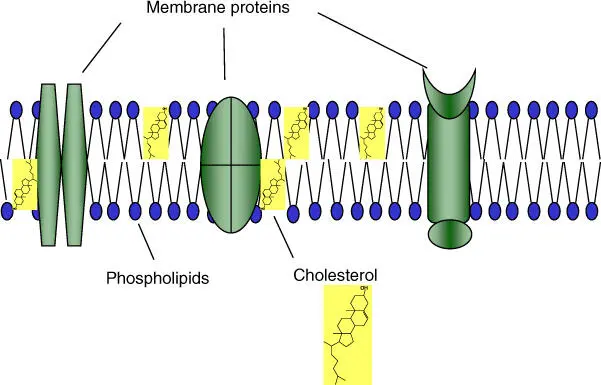
Figure 2.2 Structure of the cytoplasmic membrane. Schematic diagram of the lipid bilayer containing phospholipids, cholesterol, and membrane proteins.
Figure 2.3describes the structure of phospholipids. Of the three hydroxyl groups of the alcohol glycerol, two are linked to fatty acids (length usually 16 or 18 carbon atoms; Table 2.3), and the third is linked by an ester bond to a phosphate residue. An additional ester bond links the negatively charged phosphate residue to either an amino alcohol (choline or ethanolamine), the amino acid serine, or the sugar alcohol inositol. In the case of phosphatidylcholine(lecithin), the nitrogen atom is present as a quaternary amine and is therefore always positively charged. Phosphatidylinositolis a precursor for inositol‐1,4,5‐triphosphate (IP 3 )– an important signaling molecule in signal transduction pathways of the cell (see Section 3.1.1.3).
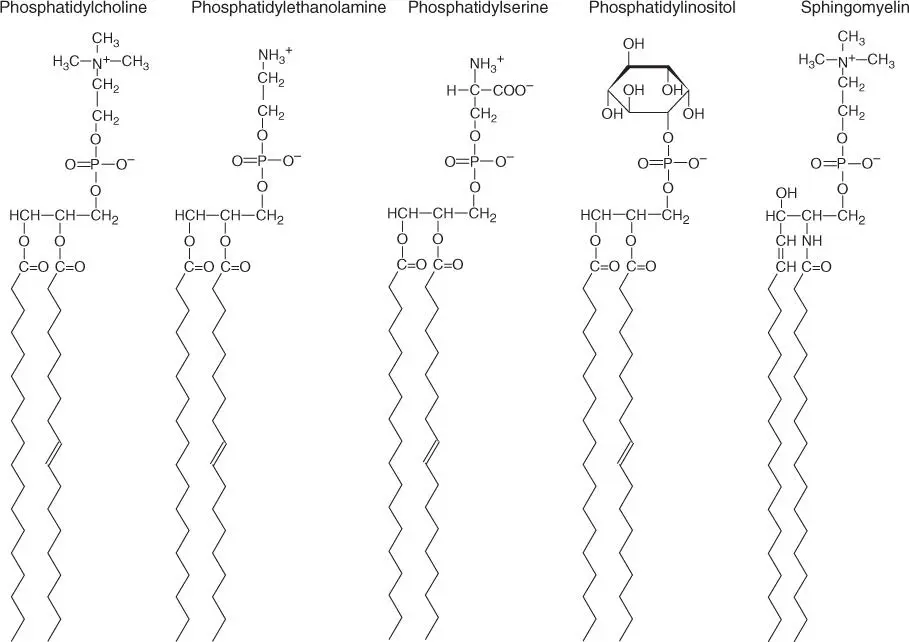
Figure 2.3 Structures of important phospholipids. Phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, phosphatidylinositol, and sphingomyelin (a ceramide).
Table 2.3 Important fatty acids in membrane lipids.
| Trivial name |
Abbreviation |
Melting temperature T m(°C) |
Structure |
| Saturated fatty acids |
| Myristic acid |
14 : 0 |
52.0 |
CH 3(CH 2) 12COOH |
| Palmitic acid |
16 : 0 |
63.1 |
CH 3(CH 2) 14COOH |
| Stearic acid |
18 : 0 |
69.1 |
CH 3(CH 2) 16COOH |
| Unsaturated fatty acids |
| Palmitoleic acid |
16 : 1 |
−0.1 |
CH 3(CH 2) 5CH=CH(CH 2) 7COOH |
| Oleic acid |
18 : 1 |
13.4 |
CH 3(CH 2) 7CH=CH(CH 2) 7COOH |
| Linoleic acid |
18 : 2 |
−9.0 |
CH 3(CH 2) 4(CH=CHCH 2) 2(CH 2) 6COOH |
| γ ‐Linolenic acid |
18 : 3 |
−17.0 |
CH 3(CH 2) 4(CH=CHCH 2) 3(CH 2) 3COOH |
| Arachidonic acid |
20 : 4 |
−49.5 |
CH 3(CH 2) 4(CH=CHCH 2) 4(CH 2) 2COOH |
Phospholipidsare amphiphilic molecules; their fatty acid residues are strongly lipophilic, while their charged head group is hydrophilic. Of the two fatty acids, one is generally unsaturated(i.e. one or more double bonds are present). As the single phospholipids constantly rotate, the fatty acid, which is kinked due to the inflexible double bond, has a significantly greater radius than that of two saturated fatty acids. This increases the fluidity of the biomembrane, and the formation of paracrystalline structuresis avoided. In bacterial or yeast cells that are exposed to different temperatures, the fluidity is constantly adjusted according to the surrounding temperatures by incorporation of phospholipids with different lengths of fatty acid residues, with or without double bonds. Also fishes, living in cold waters, have a higher content of unsaturated fatty acids than those living in warm tropical waters.
Читать дальше





![Andrew Radford - Linguistics An Introduction [Second Edition]](/books/397851/andrew-radford-linguistics-an-introduction-second-thumb.webp)








