Potential factors of causing environmental pollution such as foaming of a raw material charged and leaking of pitch from the space between the furnace body and the furnace lid in the chamber coking process can be eliminated.
Occupational health
Such a dangerous work is not required as a repair work under dangerous and hot condition frequently required for the oven wall of the carbonization chamber that is likely to be frequently damaged and a raking work under dangerous and hot condition work when plugged during discharge of coke.
Productivity
A work of unloading coke that requires a large amount of manpower becomes unnecessary, thereby improving the occupational safety and health and the productivity.
Diversity of products
The chamber coke process can produce mostly only single coke product, but the delayed coking process can produce various coke products with different properties.
Honda et al. reported that the quinolone‐insoluble (QI) components contained in coal‐tar pitch interfere in the production process of pitch needle coke (see [6]). Various methods such as elimination by hot filtration (see [10]), after dissolving in a solvent separation by filtration, centrifugation, and precipitation (see [11]), improvement of quality of a mid‐cut fraction by distillation (see [7, 12]), separation by supercritical fluid extraction (see [9]), and the like are proposed, but a method that is scaled up to industrial application is only the method in which an antisolvent is added to separate QI (see [2–4]). In this method a properly selected solvent is added to dissolve coal‐tar pitch from which QI can be easily separated. A detail of this method is described only in the patent described above. At present development activity directed to the pitch needle coke process is high in China, and many patents are applied (see [13–17]).
Calcination is performed as follows. Raw coke cut out from the coke drum contains 5–10% VM, which is eliminated by calcination. A calciner includes a retort calciner, a shaft kiln calciner, a rotary kiln calciner, and a rotary hearth calciner, but the rotary kiln calciner is generally used. The use of the rotary kiln calciner will be described as follows. A flow chart of the process using the rotary kiln calciner is illustrated in Figure 6.1.3.2. Major equipment includes a conveyor for charging raw coke, a rotary kiln, a rotary cooler, a belt conveyor, a sieving machine, and a coke silo. The calcination temperature ranges generally from 1100 to 1500 °C. What happens in each temperature zone is summarized in Table 6.1.3.5[18]. Up to 150 °C, water (7–12%) contained in raw coke is removed by drying. Raw coke is next heated to approximately 800°C to induce the evaporation of VM and the decomposition of hydrocarbons (generation of methane and hydrogen gases) in raw coke. In this step large shrinkage of coke occurs. In the calcination process to 800–1400 °C dehydrogenation and oxidation of coke occur, and the content of hydrogen in coke is reduced to 0.05% before reaching to 1400 °C. A yield in calcination is widely varied with the operational condition and the VM in raw coke but is generally approximately 80%. Calcination causes rapid densification of the carbon structure to increase the RD to 2.00–2.14. The X‐ray diffraction analysis indicates that the average crystal thickness, L c, is increased in the vicinity of approximately 900 °C and crystal growth can be observed [19] ( Figure 6.1.3.4). Particularly when needle coke is used in the aggregate of a synthetic graphite electrode, the calcination temperature in a calciner is set in a range of 1300–1450 °C, but fluctuation of the calcination temperature is preferably minimized since the amount of a pitch binder required in production of the synthetic graphite electrode is affected by the operation temperature. Excessive calcination gives the adverse effects to the production of the synthetic graphite electrode.
Table 6.1.3.5 Changes in calcination.
Source: Kurami 1973 [18]. Reproduced with permission of Wolters kluwer.
| Step |
Temperature (°C) |
Phenomenon |
| 1 |
Ambient temperature to 150 |
Drying |
| 2 |
150–480 |
Evaporation of volatile matter |
| 3 |
480–760 |
Thermal decomposition and shrinkage |
| 4 |
760–1370 |
Dehydrogenation reaction and crystallization of coke |
| 5 |
1370–1530 |
Desulfurization reaction |
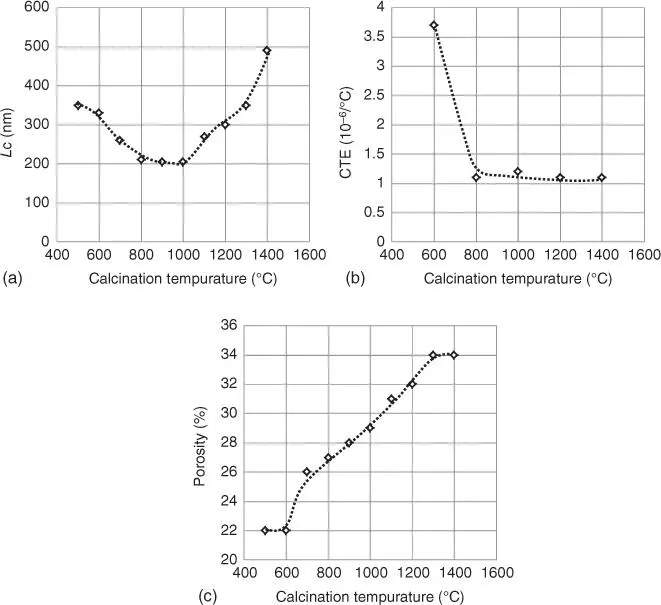
Figure 6.1.3.4 Changes in calcination ([19] extract). (a) changes of coke crystalline sixe as a function of calcination temperature. (b) changes of thermal expansion coefficient as a function of calcination temperature. (c) changes of coke porosity as a function of calcination temperature.
Important properties required for needle coke used in the synthetic graphite electrode are the coefficient of thermal expansion (CTE) to be low. CTE depends on the structure and shape of coke as a raw material. This property is almost determined in the formation of raw coke and can be little improved by the operation (and its condition) of a calciner.
PC is roughly divided into two types, amorphous coke and needle coke. The majority of amorphous coke is used as an electrode for aluminum smelting and is also used as the aggregate of the synthetic graphite block produced in the similar process to produce a synthetic graphite electrode in electric arc furnace steelmaking.
6.1.3.4.1 Aggregate of Graphite Electrode for Aluminum Smelting
Aluminum metal is produced by reducing aluminum oxide (Al 2O 3) with carbon when electrolyzing the molten salt: 2Al 2O 3+ 3C → 2Al + 3CO 2
In reduction of aluminum oxide, carbon is consumed according to the formula above. A source of carbon consumed is the anode used in the aluminum smelting furnace, and PC is used as a raw material (aggregate) of the anode.
The anode has two types, the prebake type that is calcined in advance and the Soederberg type that is calcined using waste heat in reduction, but both anodes use the aggregate (coke) and the binder pitch as a raw material. Demand in anode is increased with the growing aluminum industry, and approximately 10 000 000 t of the aggregate for an anode is used in 2009, and the majority of them use petroleum coke. A range of 100 000–200 000 t of PC is used.
Amorphous coke is also used in the process similar to the production of the synthetic graphite electrode described below, and the synthetic graphite block is also produced.
6.1.3.4.2 Aggregate for Graphite Electrode in Electric Arc Furnace Steelmaking
Since the synthetic graphite electrode in electric arc furnace steelmaking is used at high temperature, needle coke in which the crystal structure of aggregate coke is highly ordered is used. Coal tar is a mixture of polycyclic aromatic hydrocarbons, and carbonization of coal tar is anticipated to yield a hard carbon material with the highly crystalline structure. However, in coal tar there is a QI fraction, an amorphous hydrocarbon, which prevents the growth of crystals [6]. Development of the industrial process for elimination of QI first allows the production of pitch needle coke that can be used as the aggregate of the graphite electrode in electric arc furnace steelmaking (refer to Sections 1 and 3.1.2), thereby creating application of the carboniferous material.
The synthetic graphite electrode for steelmaking is produced by kneading needle coke as the aggregate with a binder pitch followed by molding, baking for carbonization of the binder pitch, impregnation of the pitch for adjustment of density, secondary baking, and graphitization. The synthetic graphite electrode for steelmaking is used in an electric arc furnace, which discharges arcs to melt steel scraps. The synthetic graphite electrode is used under severe condition, and the temperature at the electrode tip exceeds 3000 °C when used. Therefore, importance in quality of needle coke as a main raw material, particularly the low CTE, is emphasized. Pitch needle coke has the well‐developed crystal structure as compared with petroleum needle coke and has the properties in which the CTE of products obtained after graphitization is low.
Читать дальше













