Figure 1.3shows the functional relationship between the V interface/ V particlevaries and the aspect ratio of particles [13]. The aspect ratio reflects the shape of the particles, which can be divided to plate (aspect ratio <1), sphere (aspect ratio = 1), and rod (aspect ratio >1). δ represents the size of the filler, that is, the ratio of the interface thickness t to the minimum dimension size of the particle. For spherical and rod-shaped particles, δ is equal to the t / r , but δ is 2 t / h in plate-shaped particles. When the particle is microscale, δ is approximately equal to 0.01, and the particle volume exceeds the volume of the interface region in all shapes. As the particle size decreases, V interface/ V particlevalues gradually increases. When δ goes above one, V interfaceis going to exceed V particle. When the particles reach the nanoscale ( δ = 10), the interface volume is more than 10 times that of the particle. Moreover, particles with different shapes have different V interface/ V particlein the same δ . The three-dimension sphere has the highest value, followed by the two-dimensional rod and the one-dimensional plate. With the decrease of particle size, the gap becomes more obvious, and even the V interface/ V particleof spherical particles is 2 orders of magnitude larger than that of plate-shaped particles. Therefore, the addition of nanoscale fillers has a great impact on the performance of polymer in PNCs. Even if the volume fraction of fillers is very small, the resulting interface region volume will be very large.
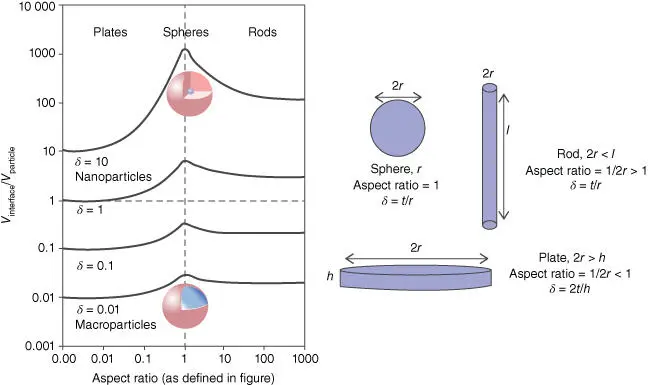
Figure 1.3 The graph on the left shows the function relationship between the ratio of interfacial volume to the particle volume ( V interface/ V particle) and the particle aspect ratio. The red shell represents the interface of particle, where the blue nucleus represents the particle. The graph on the right defines the particle aspect ratio and the ratio of the interfacial thickness to the particle size ( δ ) with different shapes ( r is radius, I is length, h is height). The interface thickness ( t ) is considered to be independent of particle size. When the particle size is reduced to less than 100 nm, the physical properties can be controlled by the volume of the interface around the particle, which is especially obvious for the sphere and rod.
Source: Winey and Vaia [13].
As the interaction between polymer and particle is strengthened in PNCs, the interparticle interface and coordination will be reflected in the macroscopic properties. Due to the nanoscale of particles, the secondary forming constituents have a very high aspect ratio of over 100. When the volume fraction is 1–5%, these fillers can reach the percolation thresholds, which refer to the critical value of the volume fraction of the packed particles that can mutate a certain physical property of the composite material system. Therefore, the mechanical and transport performances of PNCs can be greatly improved under the condition of low load nanoparticles. Especially for the conductive particles, when the volume fraction of these particles increases to a certain critical value in polymer, conductivity of the polymer suddenly increases sharply from insulator to conductor, and the change range is up to 10 orders of magnitude.
1.3 Classification of Nanoscale Fillers
So far, various types of nanomaterials have been found to be able to form PNCs with polymers. According to different applications, nanoparticles with corresponding properties can be selected into the polymer system to achieve the expected performance. In general, these nanofillers suitable for PNC applications can be mainly divided into one-, two-, and three-dimensional materials according to their different dimensions ( Table 1.1).
Table 1.1Overview of nanomaterials classified by their nanoscale dimensions.
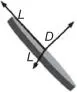
| Plate |
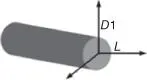
Rod |
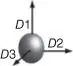
Sphere |
 |
 |
 |
| Montmorillonite clays (MMT)Nanographene platelets (NGPs)Layered double hydroxide (LDHs) |
Carbon nanofibers (CNFs)Carbon nanotubes (CNTs)Halloysite nanotubes (HNTs)Nickel nanostrands (NiNs)Aluminum oxide nanofibers (Nafen) |
Nano-silica ( n -silica)Nano-alumina ( n -Al 2O 3)Nano-silver ( n -Ag)Nano-titanium dioxide ( n -TiO 2)Nano-silicon carbide ( n -SiC)Nano-zinc oxide ( n -ZnO)POSS |
1.3.1 One-Dimensional Nanofillers
One-dimensional nanofillers are plate-like materials with one-dimensional dimensions less than 100 nm, which are usually a few nanometers thick and relatively long sheets [14]. Most one-dimensional nanofillers have unique morphology characteristics, such as nanoplate [15], nano-disk [16], nano-wall [17–23], etc., which play an important role in functional nano-devices [24, 25]. Recently, the widely studied materials are montmorillonite clays (MMT) [26], nanographene platelets (NGPs) [27, 28], ZnO nanosheets [29–31], Fe 3O 4nanosheets [30], and so on, which have excellent electrical, optical, and magnetic properties [32], and are widely used in the fields of micro–nano electronics, biosensors, and chemical engineering [33]. The one-dimensional nanofillers are common nanomaterials in electronic and thermal devices due to their shape characteristics.
1.3.2 Two-Dimensional Nanofillers
Two-dimensional fillers are the materials with two dimensions less than 100 nm, and they are mostly in the form of rods [14]. The typical two-dimensional nanomaterials are carbon nanofibers (CNFs), carbon nanotubes (CNTs), halloysite nanotubes (HNTs), nickel nanostrands (NiN S), and aluminum oxide nanofibers (Nafen). In addition, the most common two-dimensional nanofillers in PNCs are nanotubes [34], plant fibers [35–39], nanowires [40], carbon fibers [41–44], oxides [45–55], graphene [56, 57], molybdenum disulfide (MoS 2) [58], and hexagon boron nitride ( h -BN) [59]. Compared with one- and three-dimensional fillers, two-dimensional fillers have better flame retardancy and striped characteristic, resulting in wide applications in the fields of catalysis, electronics, optics, sensing, and energy [3, 26, 60–62].
1.3.3 Three-Dimensional Nanofillers
Three-dimensional nanofillers are nanomaterials with three dimensions on the nanometer scale, so they are mostly spherical or cube-shaped [63], which is also commonly referred to zero-dimensional particles. The most common three-dimensional fillers are polyhedral oligomeric silsesquioxane (POSS), nanosilicon, nanometal particles, nanometal oxides, and quantum dots (QDs) [33, 64]. Among them, metals and metal oxide nanoparticles have the advantages of high stability, catalytic activity, and easy preparation, and they are often used in the fields of catalysis [65], purification [66–69], coatings [70–74], and biological fields [75, 76], together with various polymers. One-, two-, and three-dimensional nanofillers all have various special properties, and will ultimately promote the remarkable performance of PNCs by loading in compatible polymers.
Читать дальше
















