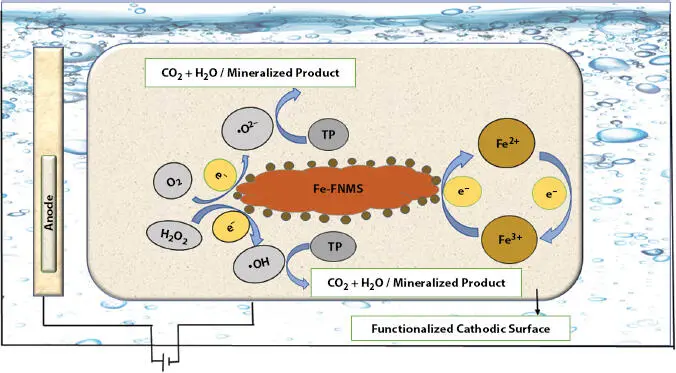
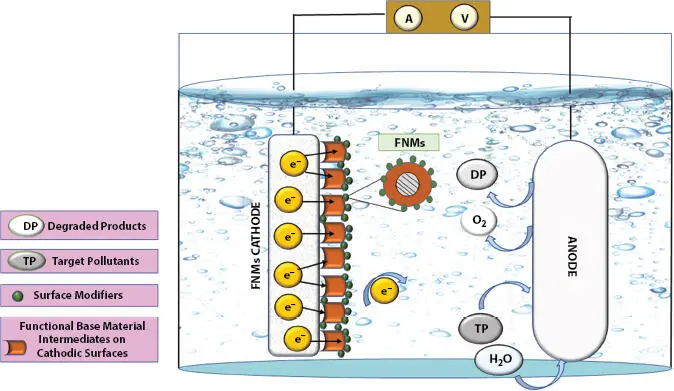
Fenton’s redox chemistry employs ·OH released between the reacting species (H 2O 2+ Fe 2+) for the decomposition of target pollutants (TPs), where electro-Fenton (EF) or photoelectro Fenton (P-EF) have prominent roles. Hetero-EF (H-EF) utilizes solid nanocatalyst as a supporter for reducing H 2O 2→ ·OH. The disadvantage of small pH range (acidic) is overcome by solid supporters when used. The effluents released into the water system have a wide range of pH [47]. Micro-porous/meso-porous FNMs offer best solutions for degrading OPs in the water bodies. Research communities are focusing on this segment for protection of environmental crises using Fe/other transition metal/metal oxides as cathodic FNMs in H-EF methods.
Cathodic FNMs are got by (i) uni/multi step synthesis of low-density porous-solids (C aerogels), (ii) modified conducting FNMs with Fe, and (iii) carbonaceous solids supported with Fe or other components as FNMs [48–50]. Formation of sludge as Fe-hydroxides, as in normal Fentons, is retarded or inhibited, thus improving the efficiency and availability of catalyst for its activity. Hence, less energy utilization and a cost-effective approach is favored. Similarly, reusability and recyclability for many trials were observed while using cathodic FNMs of Fe 2O 3/N-C by [51] and Fe-Cu-C aerogel [52]. However, Fe when strengthened with other metals (transition) embedded in it, results in a redox reaction with catalytic decomposition, and is favored with the increase in efficacy of the electrocatalytic system to bring about degradation of TPs [53–55]. A figurative description of the functionalized catalytic activity is shown in Figure 1.3.
In a typical report of Cui, L. et al ., MO decomposition by H-EF was proved to be accelerated by FNM - Fe 3O 4/MWCNTs, when prepared by solvothermal process. Degradability of the TP was noted to be 90.3% (3 h) with reusability to 5 runs, at pH (3). This system with two compartments of FNM membrane required no external additives, but had a potency in green wastewater treatment techniques [56]. Zhao, H. et al . reported that Fe 3O 4@Fe 2O 3/ACA (activated C aerogel) as cathodic in this EF routine degraded (90%) of OP-pesticide imidacloprid (30 min) and TOC (60 min) in pH range of (3–9) [57]. Haber-Weiss model inferred that Fe 2+aided the decomposition of peroxide to form ·OH. ·OH and ·O 2−contribute for the degradation of OP. Mesoporous FNMs MnCo 2O 4-CF (C felt) as cathodic EF with excellent porosity and large modified surface area prepared showed a powerful degrading capacity for CIP (100%) an antibiotic in 5 h and TOC (75%) in 6 h [58]. Mn 2+/Mn 3+, Co 3+/Co 2+with e −transfers enhanced peroxide decomposition to form ·OH and ·OOH required for five cycles degradation.
| FNMs as catalyst | Type | Year |
Process | Current/Voltage |
Parametric expressions |
Solution evolved (% degradation) | Reusable cycles |
Remarks |
Ref. |
| BGA-GDE | EF | 2019 |
Hydrothermal | 4.5 mA cm −2 |
pH (3–9) | 60 min |
BPA (~89.65%) | 5 TOC (~90%) | 5 |
·OH | pseudo-1 st-order kinetics |
[62] |
| RGO-Ce/WO 3NS/CF | EF | 2018 |
Hydrothermal | 300–400 mA |
pH (3) | 1h |
CIP (100%) | 5 |
·O 2−, H 2O 2, ·OH | Ce-WO 3improved adsorption |
[63] |
| ACF-HPC | EF | 2019 |
Hydrothermal, carbonization | (16, 20, 24) mA cm −2 |
pH (3, 7, 9) | 40, 180 min |
Phenol (93.8%) | 5TOC (85.7%) | 5 |
Enhanced formation of H 2O 2, ·OH | Low-cost |
[64] |
| Fe-C/PTFE | H-EF | 2015 |
Ultra-sonification | 100 mA |
pH (6.7) |120 min |
2,4-DCP (95%) | |
pseudo-1 st-order kinetics | promoters: H 2O 2, ·OH | Cheap |
[65] |
| N-C (NF) as (c PANI/GF2) | EF | 2019 |
Carbonization (PANI) |−0.6 V |
pH (3) |180 min |
Mineralization (42%) | Florfenicol (99%)| Phenol (85%) | MO (100%) | 5 |
Activation: H 2O 2→ ·OH |
[66] |
| FeO x/NHPC750 | H-EF | 2020 |
Hydrothermal, carbonization | (−3.30, −4.42, −3.77) mA cm −2| −0.6 V |
pH (6) |90 min |
ATZ (96%) | Rh B (99%) | 2,4-DCP (99%) | Sulfamethoxazole (95%) | Phenol (99%) | 5 |
Cleavage of O-O bond | Assists H 2O 2| Fe 2++ O 2→ Fe 3++ ·O 2− |
[67] |
| (Co, S, P)/MWCNTs | P-EF |2019 |
Hydrothermal | 40 mA cm −2 |
pH (3) |360 min |
Bronopol (100%) |TOC (77%) | 3 |
Contributors: sunlight, ·OH, BDD ( ·OH) | |
[68] |
| Mn/Fe@porous C (PC)-CP cathode | H-EF | 2019 |
Carbonization | 40 mA |
pH (2–8) |120 min, 240 min |
TCS (100%) | TOC (~57%) | 6 |
Regeneration: Fe 2+/Mn 2+/3+| e-transfer: Fe 2+/ 3+, Mn 2+/ 3+/ 4+,pseudo-0-order kinetics |
[69] |
| 3DG/Cu@C | H-EF | 2020 |
Hydrothermal, calcination | 30 mA |
pH (3–9) | 150 min |
Rh B (100%) | CIP (100%) | 2,4-DCP (100%) | PCA (89.8%) | BPA (96.1%) | CAP (82.6%) | 5 |
Contributors: ·OH, ·O 2−| e- transfer: Cu 2+/ + |
[70] |
| C felt/Fe-Oxides | H-EF | P-EF | 2016 |
Electro-deposition | 21.7 mA cm −2 |
pH (3) |120 min |
MG (98%) | 10 |
·OH, BDD - activators | UVA | pseudo-1 st-order kinetics | |
[71] |
| (N-G@CNT | EF | 2108 |
Hydrothermal | variable | −0.2 V |
pH (3) |180 min |
DMP (100%) | 20TOC (40.4%) |
Fe 2++ H 2O 2→ Fe 3++ e −| pseudo-1 st-order kinetics |
[72] |
| F-rGO/SS membrane | EF | 2019 |
Electrophoretic deposition | 170 mA | −0.5 V |
pH (3) | |
PCM (37%) | 5 |
e -transfer-enhanced by rGO | low-cost membrane |
[73] |
| G-CNT-CE | EF | 2014 |
Electrophoretic deposition | 0.18 A |
pH (3) |210 min |
Acid Red 14 (91.22%) |Acid Blue 92 (93.45%) |
pseudo-1 st-order kinetics | pseudo-2 nd-order kinetics |
[74] |
| Fc-ErGO | EF | 2018 |
Electrochemical | −1.5 V | (−0.75, −1.0, −1.5, −2.5) V |
pH (3) | 15 min | pH (7) | 120 Min |
CIP (99%) | 5 |
·OH | pseudo-1 st-order kinetics |
[75] |
| FeOCl-CNT | EF | 2020 |
Thermal-induced | −0.8 V |
pH (wide) | |
TC (99.5%) | |
Fe 3+/Fe 2+| H 2O 2+ ·OH → H 2O + ·OOH |
[76] |
| 3D GA/Ti wire | EF | 2018 |
Hydrothermal | [0 – (−95.5)] mA cm −2 |
pH (2) |120 min |
EDTA-Ni (m73.2%) | 5 |
π-π interaction | pseudo-1 st-order kinetics |
[77] |
H-EF system with Fe oxides surrounded by Cu and N on HPC (hollow porous C) as cathodic FeO x/CuN xHPC was inferred to give a good degradability for phenol (100%/90 min/variable pH) and (81%/120 min/pH6). Slow redox reaction (Fe 2+/Fe 3+) favored e −movement, and formation of ·OH, that were essentials for degradation in this ambient condition [59]. In a similar fashion a three-layered H-EF catalyst as FMN “CFP@PANI@Fe 3O 4” engineered using electrodeposition-solvothermal method was proven for the removal of 4-NP (100%/60 min/4 runs) at an acidic pH (3) and TOC (51.2%/7 runs) at the same pH [60]. Enrichment of electrocatalytic capacity was attributed to formation of Fe 3O 4on the functionalized surface of the conducting layers. Wang, Y. et al . fabricated γ-FeOOH GPCA cathodic EF-catalyst for experimenting the degradability of the antibiotic sulfamethoxazole (~90%/5runs). Twelve degraded products by, hydroxylation, isomerization, and oxidation reactions were identified using chromatographic trials [61]. Table 1.1depicts trials developed by some research personalities.