1.4 Hetero Photo-Fenton as FNMs
Organic effluent’s water management is achieved well, in normal environmental pre-requisites of pH, where the hetero-photo Fenton has a good stability and reusability for notable cycles. Photo-Fenton (PF) catalytic redox reaction may utilize membrane percolation and magnetic purification to effectually decompose OPs into CO 2and H 2O. Semiconductors, phosphates/oxides/oxyhalides/sulfides/molybdates/tungstates/vanadates of transition and other metals, g-C 3N 4, G, GO, QDs, and MOFs have fascinated the researchers to a larger degree, for probing a simplified and a cost-effective FNMs [78, 79]. Excellent features with required bandgap, got by photo-excitation in an environmentally friendly way, of the materials with notable lattice parametric change, safe guard water system by nanocatalytic action. Figure 1.4is a graphical representation of FNMs as PF catalyst for water resource management.
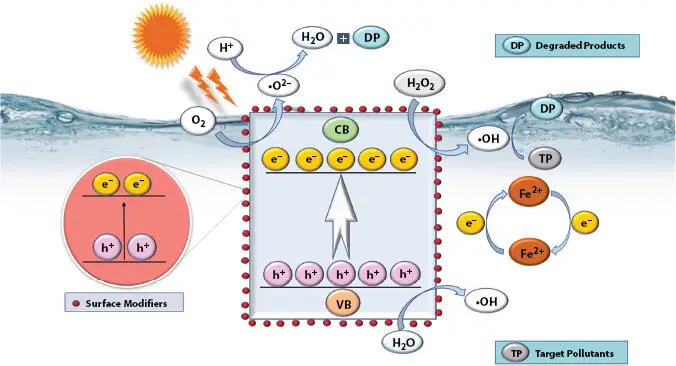
Figure 1.4 Functionalized nanomaterials as photo-Fenton catalyst for water resource management .
1.4.1 Heterogenous-Fentons-Based FNMs
FNM PAN with EDC as catalyst was used to study the regeneration efficiency of the material in use, by the experimentalist. Further, the regenerated catalytic reaction as heterogenous Fenton (HF) was found to be a better option for degrading RO-16 [80]. Living species vulnerable to the revelation of pharmaceutical organic lteftovers in the water system causing ecological barriers was the point of attack by reporters Wan, Z. et al . [81]. Fe 3O 4-Mn 3O 4/RGO synthesized by polyol and impregnation processes worked well as a HF-like catalyst with the actively formed OH, decomposed SMT (sulfamethazine-drug) (99%/50 min) effectively in a water solvent. Authors Zhou, L. et al ., forecasted that the drawbacks due to catalytic reactions can be knocked off by using MPCMSs [82]. C micro-spheres protected Fe 3O 4NM from oxidation, while degrading MB. Formation of ·OH supported this HF reaction by H 2O 2and NH 2OH.
1.4.2 Photo-Fentons-Based FNMs
Hetero Photo-Fenton (H-PF), an interface between Fenton and photocatalysis, assisted by photon from solar or visible has powerful synergistic properties. PF utilizes the e −’s got from the reaction Fe 3+/Fe 2+through oxidation to aid and activate e −transfer to ·OH from H 2O 2in the entire redox process. The scavenging radicals contribute to practical utilization in a big factor for protecting water bodies.
Fe oxNPs/D3 (diamond NP) that worked well as a H-PF catalyst was effective in degrading and decomposing phenol and H 2O 2, respectively, under an ambient condition. Later, was proved to be a better alternative when in comparison with its analogous as per reports of Espinosa, J.C. et al ., where phenol acts as h +quencher and diamond NP as surface releaser of ·OH favors the optimized reaction [83]. Upgraded new water treatment competencies are scaling up in saving the water resources. For instance, a low-cost valuable functionalized M (magnetite)/PEG/[(FeO (Iron III oxlate)/FeC (Iron III citrate)] showed high catalyst action for a quick disposal and degradation of BPA. Later, the evidenced PF catalyst when on exposing the chosen probe with UV-A light/H 2O 2, had a hierarchical degradation as (M/PEG/FeO) (15 min) > (M/PEG/FeC) > (M/PEG) [84]. In one of their work, a comparative study of MB degrading effect by the synthesized supports of Ni foam (NiF) and Ceramic foam (CM) was done by the authors, whose reports infer that the order of decomposing capacities were: (NiF/TiO 2) > (CM/TiO 2) > (NiF/Bi 2WO 6) > (CM/Bi 2WO 6). In the same manner, decomposition of Rh B was studied using NF/TiO 2for PF reactions [85].
In a different situation, reporters Bui, V.K.H. et al ., revealed that Mg-AC (Mg amino-clay) with its versatility and unique characteristic, along with other 2D resources, have fascinated researchers [86]. Hence, Mg-AC finds its place and with a commendable performance in various fields especially in water resources. The resourceful material MgAC-Fe 3O 4/TiO 2works best for PF catalytic decomposition of MB (93%) (20 min), where ·OH and ·O 2−formed are promoters for the redox-reaction. Cost-effective and potential approach with Fe-HPAN (carboxyl) functionalized beads developed by researchers was exposed for an effective PF catalytic reaction. Their results showed a better degrading capacity of 99.78% for TOC and 91.68% for p-nitrophenol removal and profitable reutilization was supported by the mesopores present in the FNM [87].
Rice-shaped starch functionalized iron (III)-oxyhydroxide got by green methods using akageneite/goethite had an improved HF and PF catalytic properties obeyed a pseudo-0-order kinetics. FNM was effective in decomposing the OP p-nitrophenol and MO into CO 2+ H 2O to protect the water bodies [88]. In a similar trial, sulfate-functionalized S-Fe 2O 3/TiO 2NT prepared by solvothermal/impregnation process was proven to be a good candidate for PF catalytic run to discharge the color of X-3B by adopting pseudo-1 st-order kinetics. Notable degradation was observed at pH (4.0) with X-3B (95.7%) lost. Reusability was for four cycles, where ·OH | ·OOH played their role for degrading the pollutant [89]. Similarly, reporters Banić, N. et al . inferred that Fe/TiO 2(TiO 2as host) as PF catalyst could effectively degrade the pesticide thiacloprid by UV irradiation, which was successful for three trial runs [90]. Other significant exposures to preserve the water system using PF and photo-Fenton–like (pF) have been detailed in the segments to come in Table 1.2.
Table 1.2 Photo-Fenton (PF)/Photo-Fenton–like (pF) catalyst as FNMs.
| FNMs as catalyst | Year |
Process |
Irradiation Source | |
Parametric expressions |
Solution evolved (% degradation) | Reusable cycles |
Remarks |
Ref. |
| TiO 2/Schwertmannite | PF | 2019 |
Solvent-free milling |
Sunlight |
pH (4) | 60 min. |
Rh B (100%) | 4 |
TiO 2→ Sh + e −H 2O 2+ e −→ ·OH |
[91] |
| TiO 2/Fe 2TiO 5/Fe 2O 3| PF | 2017 |
Ion-exchange |
Visible light > 420 nm |
pH (4.0/7.0) | 120 min | 60 min |
MO (100%) | Phenol (100%) | 10 |
OP+ ·OH → CO 2+ H 2O |
[92] |
| A-TiO 2/R-TiO 2/α-Fe 2O 3| PF | 2020 |
Aerosol spray |
UV - 365 nm |
pH (8) | 5–30 min |
MB | TOC | 5 |
O 2/ ·O 2−| low dose H 2O 2 |
[93] |
| TiO 2-GO-Fe 3O 4| PF | 2019 |
Ultrasonic |
Visible light |
pH (3) | 120 min |
Amoxicillin (90%) | 4 |
Fe 3+→ Fe 2++ e − |
[94] |
| FeN x/g-C 3N 4| PF | 2019 |
Ball milling |
Visible light |
pH (neutral) | |
MB |MO |Rh B |Phenol | (variable %) | 4 |
H 2O 2+ e −→ 2 ·OH |
[95] |
| 0D Fe 2O 3QDs/2D g-C 3N 4| PF | 2020 |
Thermal polymerization |
Visible light |
pH (3–7) | 20 min |
4-NP (90%) | 5 |
Fe 3+→ Fe 2++ e −OH+H ·2O2→ ·OOH+H 2O |
[96] |
| α-Fe 2O 3/g-C 3N 4| PF | 2020 |
Hydrothermal |
Solar light |
pH (neutral) | 90 min |
Rh B (96%) | 5 |
Binding Energy (284.8 eV) ·O 2−/h +. Fe 3+→ Fe 2++ e − |
[97] |
| Zn0.94 Fe 0.04S/g-C 3N 4| PF | 2020 |
Microwave| Hydrothermal |
Solar light |
pH (6.1) | 60 min |
4-NP (96%) |TOC (55.4%) | 5 |
CB favors e −transfer Fe 3+→ Fe 2++ e − |
[98] |
| Cu-FeOOH/CNNS(g-C 3N 4) | PF | 2018 |
Simple thermal |
Solar light |
pH (4.8-10.1) | 40 min | pH (low) |
MB | Rh B | MO | CR | 4-NP | TC | ~90% (OP) | 10 |
H 2O 2+ e −→ 2 ·OH ·O 2−| ·OH (Scavengers) | pH (low) efficient |
[99] |
| (Fe-CS/MMTNS | PF | 2020 |
Sol gel (3 Step) |
Visible light |
pH (3,6,10) | 2 h |
MB (55.81%) | 5 |
·OOH | ·O 2−| activators | n → π*| π → π* - transition |
[100] |
| Fe 0)/MnO x/BiVO 4| PF | 2019 |
Hydrothermal | Photo-deposition |
Visible light |
pH (acidic) | 30 min |
2,4-di-CP (95.4%) | BPA (91.4%) | 4 |
Rate of reaction ·OH > h +> ·O 2−| bandgap (2.10 eV) |
[101] |
| GO/MIL-88A(Fe) | PF | (2020) |
Vacuum-filtration |
Visible light |
- | 40 min |
MB (98.81%) | BPA (97.27%) |12 |
·OH > ·O 2−>>h +| Major part in degradation |
[102] |
| Fe-POM/CNNS- Nvac | PF | 2020 |
Self-assembly |
Visible light < 420 nm |
- | 18 min |
TCH | ATZ | ALA |MO | 4-CP | (~96.5%) | 4 |
Contributors h +| 1O 2| ·OH | ·O 2−| |
[103] |
| QDs-Fe/G | NRs-Fe/G | NSs-Fe/G | PF | 2015 |
GMSA |
Visible light |
pH (neutral) | 30 min |
Phenol | RhB | - |
Novel green synthesis | Scavenger - ·OH |
[104] |
| 3D FeO (OH)-rGA | PF | 2018 |
Facile method-Hummer’s |
Visible light |
pH (neutral) | 6 h |
4-CP | 2,4,6-triCP | BPA | (80%) | 10 |
Activation of ( ·OH) | π-π interaction |
[105] |
| CQDs/α-FeOOH | PF |2020 |
Hydrolysis |
Visible light < 420 nm |
pH < 7 | 60 min |
TC (90%) | 5 |
Activation of ( ·OH) | π → π* - transition |
[106] |
| Fe 3O 4(MPs)/(HA) Humic acid | pF | 2020 |
Co-precipitation |
Sunlight |
pH (<4) | 60 min |
CBZ | IBP |BPA| 5-TBA | 4-CP | |
Fe 3+→ Fe 2++ e −urban wastewater used |
[107] |
| Fe 3O 4@void@TiO 2| pF | 2017 |
Sol-gel |
UV light |
pH (3) | variable |
TC (100%) | 5 |
Fe 3+→ Fe 2++ e − |
[108] |
| FeCu@Fe 2O3-g-C 3N 4| pF | 2020 |
Calcination |
Visible light |
pH (3–11) | 6 h |
Aniline (80%) | 4 |
Degrading efficiency is high for FeCu-CN | |
[109] |
| Fe 3O 4@MIL-100w | pF | 2015 |
Solvothermal |
Visible light |
pH (3–6.5) | 120 min |
MB (~99%) | 20 |
Activation of ( ·OH) |
[110] |
1.5 Photocatalysts as FMNs
Читать дальше













