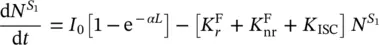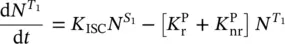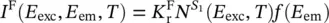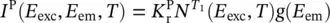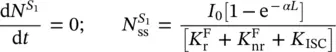This scheme is known as the Jablonski diagram [2, 5]. The system is assumed to be at a temperature T characterized by a thermal energy much lower than the vibrational energy of nuclei: kT ≪ ℏω , so only the lowest vibrational levels could be populated in thermal equilibrium; as an extreme case, it could be assumed that T = 0 K. The interaction with an electromagnetic field of opportune frequency gives rise to the absorption process in which a photon is lost by the field and the electron is promoted from the S 0to the S 1state. This effect is in a typical timescale much faster than any nuclear motion and can be assumed to occur in 10 −15s [5]. As stated above, the nuclei are in a fixed position and with given momentum during this process; as a consequence, if the starting vibrational state at low T is the ground vibrational state, the arrival vibrational state, in the electron excited state S 1, is not necessarily the lowest vibrational energy level (this depends on the Franck–Condon factor). This is sketched by the tip of the absorption arrow pointing to a high vibrational level in S 1. Successively, the nuclei relax and release their vibrational energy, reaching the lowest vibrational level (marked by the white arrow R ), in a time typical of nuclear vibration: 10 −12s [7, 14]. On reaching the lowest vibrational level in S 1, the system could relax to the S 0state through coupling to its highly excited vibrational levels and the internal conversion (IC, dashed arrow) process and without energy release to the electromagnetic field (non‐radiative relaxation, heating of the molecule). It is also possible that the system relaxes to the S 0state by emitting a photon. This process is called fluorescence and the overall permanence in the S 1state of an ensemble of similar molecules gives the lifetime of this emission process. A typical timescale starting from 10 −9s characterizes this process. This transition is spin allowed and the short lifetime is a characteristic feature. It is worth noting that also in this case the arrival vibrational state in S 0is determined by selection rules and could not be the ground vibrational level. Another pathway from the S 1state involves the molecular vibrations and the spin–orbit coupling [2, 5]. This process enables to change the spin state of the electrons, giving a change from singlet ( S 1) to triplet ( T 1) state. This interaction process is known as intersystem crossing (ISC, short‐dashed arrow) and gives rise to a non‐radiative relaxation between excited states. Once in T 1, where the vibrational state could be different from the ground state, a vibrational relaxation occurs to the ground vibrational level (white arrow). Successively, the system could reach the S 0state through ISC relaxation to its high‐energy vibrational states (short‐dashed arrow). Furthermore, a transition to S 0could occur with the emission of a photon by the phosphorescence phenomenon. In this case, the permanence in the excited state T 1determines the lifetime of this emission process that typically is in a timescale larger than 10 −4s. It is observed that this transition is spin forbidden, since a passage from triplet to singlet state occurs, and the related lifetime is much longer than for fluorescence. Overall, the ISC and IC processes are related to vibrations of the molecule and are known as phonon‐assisted processes. The presence of vibrations is related to the temperature of the system and an Arrhenius law is assumed for these processes [2, 5]. In detail, the rate of the intersystem crossing process, K ISC, is given by
(1.103) 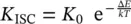
where K 0is a pre‐exponential factor taking into account entropic‐statistical factors, Δ E is the activation energy of the process, and k is the Boltzmann constant.
The Jablonski diagram is useful to describe the overall emission features of a system and to schematize the energy levels distribution and their dynamics aspects.
1.2.5 Excited States Rate Equations
To go deeper in the emission features, a simplified Jablonski diagram for the transition processes of electrons among vibronic states can be considered, joining the representations reported in Figure 1.4(bottom) and Figure 1.6. The singlet energy levels S 0and S 1are connected by the absorption process and by the radiative fluorescence emission from S 1with rate 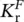 , and the non‐radiative decay with rate
, and the non‐radiative decay with rate 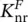 . The T 1state is populated by the intersystem crossing with rate K ISC,and it is connected to the S 0state by the phosphorescence emission, with rate
. The T 1state is populated by the intersystem crossing with rate K ISC,and it is connected to the S 0state by the phosphorescence emission, with rate 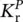 , and by the non‐radiative process with rate
, and by the non‐radiative process with rate 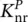 . Under light excitation, it is possible to describe the time‐dependent population of the excited singlet,
. Under light excitation, it is possible to describe the time‐dependent population of the excited singlet, 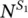 , and triplet,
, and triplet, 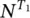 , states by the rate equations [15, 18]:
, states by the rate equations [15, 18]:
(1.104) 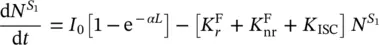
(1.105) 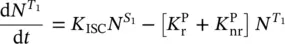
where the absorbed light through a sample of thickness L , giving transitions from S 0to S 1, has been introduced based on Eq. (1.5). It is observed that the emission from the excited states depends on their population; so, it can be stated that for the fluorescence the emitted light is given by
(1.106) 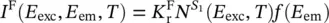
where the excitation, E exc, and the emission, E em, energies have been introduced together with the temperature dependence and a lineshape f ( E em) including the homogeneous and inhomogeneous distributions of levels [18]. Analogously, for phosphorescence, it is found that
(1.107) 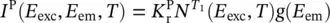
where the lineshape for the triplet to singlet emission of phosphorescence, g ( E em), has been inserted. In the stationary state (ss), it is found that
(1.108) 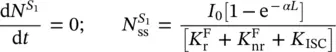
Читать дальше

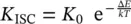
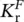 , and the non‐radiative decay with rate
, and the non‐radiative decay with rate 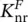 . The T 1state is populated by the intersystem crossing with rate K ISC,and it is connected to the S 0state by the phosphorescence emission, with rate
. The T 1state is populated by the intersystem crossing with rate K ISC,and it is connected to the S 0state by the phosphorescence emission, with rate 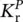 , and by the non‐radiative process with rate
, and by the non‐radiative process with rate 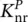 . Under light excitation, it is possible to describe the time‐dependent population of the excited singlet,
. Under light excitation, it is possible to describe the time‐dependent population of the excited singlet, 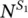 , and triplet,
, and triplet, 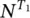 , states by the rate equations [15, 18]:
, states by the rate equations [15, 18]: