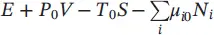The second law also states that the entropy in the universe always increases. As mentioned before, entropy is a measure of degree of disorder, and every process happening in the universe increases the entropy of the universe to a higher level. The entropy of a state of a system is proportional to (depends on) its probability, which gives us an opportunity to define the second law in a broader manner as “the entropy of a system increases in any heat transfer or conversion of energy within a closed system.” That is why all energy transfers or conversions are irreversible. From the entropy perspective, the basis of the second law is the statement that the sum of the entropy of a system changes and that of its surroundings must always be positive. Recently, much effort has been invested in minimizing the entropy generation (irreversibilities) in thermodynamic systems and applications.
Moran and Shapiro [3] noted that the second law and deductions from it are useful because they provide a means for
predicting the direction of processes;
establishing conditions for equilibrium;
determining the best performance of thermodynamic systems and applications;
quantitatively evaluating the factors that preclude the attainment of the best theoretical performance level;
defining a temperature scale, independent of the properties of the substance; and
developing tools for evaluating some thermodynamic properties, for example, internal energy and enthalpy, using available experimental data.
Consequently, the second law is the linkage between entropy and the usefulness of energy. The second law analysis has found applications in a wide variety of disciplines, for example, chemistry, economics, ecology, environment, and sociology, far removed from engineering thermodynamics applications.
1.4.20 Reversibility and Irreversibility
These two concepts are highly important to thermodynamic processes and systems. Reversibility is defined by the statement that only for a reversible process can both a system and its surroundings be returned to their initial states. Such a process is only theoretical. The irreversibility during a process describes the destruction of useful energy or availability. Without new inputs, both the system and its surroundings cannot be returned to their initial states because of the irreversibilities that have occurred, for example, friction, heat transfer or rejection, and electrical and mechanical effects. For instance, an actual system provides an amount of work that is less than the ideal reversible work, so the difference between these two values gives the irreversibility of that system. In real applications, there are always such differences, and therefore real processes and cycles are always irreversible.
Table 1.4 Relations among essergy, availability, exergy, and free energy.
Source: Szargut et al. [4].
| Name |
Function |
Remarks |
| Essergy |
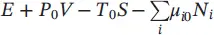 |
Formulated for the special case in 1878 by Gibbs and in general in 1962, and changed from available energy to exergy in 1963, and from exergy to essergy (i.e. essence of energy) in 1968 by Evans |
| Availability |
E + P 0 V − T 0 S − ( E 0+ P 0 V 0− T 0 S 0) |
Formulated by Keenan in 1941 as a special case of the essergy function |
| Exergy |
E + P 0 V − T 0 S − ( E 0+ P 0 V 0− T 0 S 0) |
Introduced by Darrieus in 1930 and Keenan in 1932; called the availability in steady flow by him, and exergy by Rant in 1956 as a special case of essergy |
| Free energy |
Helmholtz: E – TS |
Introduced by von Helmholtz and Gibbs in 1873 as the Legendre transforms of energy to yield useful alternate criteria of equilibrium, as measures of the potential work of systems representing special cases of the essergy function |
| Gibbs: E + PV – TS |
Exergy is defined as the maximum amount of work (also called availability , see Table 1.4) that can be produced by a stream of matter or energy (heat, work, etc.) as it comes to equilibrium with a reference environment. Exergy is a measure of the potential of a flow or system to cause change as a consequence of not being in complete stable equilibrium relative to a reference environment. For exergy analysis, the state of the reference environment, or the reference state, must be specified completely. This is commonly done by specifying the temperature, pressure, and chemical composition of the reference environment. Exergy is not subject to a conservation law. Rather exergy is consumed or destroyed because of irreversibilities in any process. Table 1.5compares energy and exergy from a thermodynamics point of view.
As pointed out by Dincer and Rosen [5, 6], exergy is a measure of the usefulness, quality, or potential of a flow or system to cause change, and is therefore a type of measurement of the potential of a substance to impact the environment.
Exergy analysis is a method that uses the conservation of mass and conservation of energy principles together with the SLT for the design and analysis of systems and processes. The exergy method can be suited for furthering the goal of more efficient energy‐resource use, for it enables the locations, types, and true magnitudes of wastes and losses to be determined. Therefore, exergy analysis can reveal whether or not, and by how much, it is possible to design more efficient energy systems by reducing the sources of inefficiency in existing systems. In the past, exergy was called essergy, availability, and available energy . Table 1.4lists some relations among essergy, availability, exergy, and free energy.
From the point of view of energy and exergy efficiency, it is important to note that if a fossil fuel‐based energy source is used for a low‐temperature thermal application like space heating or cooling, there would be a great difference between the corresponding energy and exergy efficiencies, perhaps by as much as 50–70% for the energy efficiency and 5% for the exergy efficiency [7]. One may ask why, and to address that question, we provide the following:
Table 1.5 Comparison between energy and exergy.
| Energy |
Exergy |
| • Dependent on the parameters of matter or energy flow only, and independent of the environment parameters |
• Dependent both on the parameters of matter or energy flow and on the environment parameters |
| • Has values different from zero (which is equal to mc 2in accordance with Einstein's equation) |
• Equal to zero (in dead state by virtue of being in equilibrium with the environment) |
| • Guided by the first law of thermodynamics for all processes |
• Guided by the first and second laws of thermodynamics for reversible processes only (in irreversible processes, it is destroyed partly or completely) |
| • Limited by the second law of thermodynamics for all processes (including reversible ones) |
• Not limited for reversible processes owing to the second law of thermodynamics |
| • Conserved in all processes |
• Not conserved in all processes |
High‐quality (e.g. high temperature) energy sources such as fossil fuels are often used for relatively low‐quality (e.g. low‐temperature) processes like water and space heating or cooling.
Читать дальше