Mohamed N. Rahaman - Materials for Biomedical Engineering
Здесь есть возможность читать онлайн «Mohamed N. Rahaman - Materials for Biomedical Engineering» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Materials for Biomedical Engineering
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Materials for Biomedical Engineering: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Materials for Biomedical Engineering»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
A comprehensive yet accessible introductory textbook designed for one-semester courses in biomaterials Materials for Biomedical Engineering: Fundamentals and Applications
Materials for Biomedical Engineering: Fundamentals and Applications
Materials for Biomedical Engineering — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Materials for Biomedical Engineering», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Source: From Liu et al. (2013) / with permission of Elsevier.
These data illustrate features that are significant in the design of brittle materials for use in biomedical applications that are subjected to significant stresses, such as healing large defects in the long bones of the human limbs. At a specific applied stress, the bioactive glass specimens are more reliable in compression than in bending due to their higher m and σ ovalues in compression. This may be attributed to the higher volume of the specimen subjected to a tensile stress in bending. For example, the maximum compressive stress on the human femur in walking or running is estimated at less than ~10 MPa. Based on the data in Figure 4.15, the probability P fthat one of these bioactive glass specimens will fail under a compressive stress of 10 MPa is estimated at less than 10 −6, that is, less than one in a million specimens is predicted to fail. On the other hand, for the same stress (10 MPa) in bending, the probability of failure P fis 0.3, or approximately 1 in 3 specimens will fail.
The data in Figure 4.15also show that the Weibull parameters for β‐tricalcium phosphate are considerably lower than those of hydroxyapatite that, in turn, are lower than those of the bioactive glass. Due to its low mechanical reliability, β‐tricalcium phosphate should not be used in any application that is subjected to even a low compressive stress. In general, bioactive ceramics such as bioactive glass, hydroxyapatite and β‐tricalcium phosphate degrade in the biological environment in vivo ( Chapter 7) and lose a significant fraction of their strength with time, an important factor that is not represented by the data in Figure 4.15.
4.4.3 Designing with Polymers
In designing with polymers, the property of viscoelasticity must be taken into account. For applications in which a tensile load is present, for example, data for the tensile strength and creep modulus over the appropriate range of conditions such as stress, time scale, and temperature are relevant. If the polymer degrades in vivo , the effects of environmental conditions should also be taken into account. Designing to avoid yielding of the polymer is often straightforward once the time and temperature dependence of the yield strength is accounted for. In comparison, designing to avoid brittle fracture is more difficult due to the presence of flaws such as microcracks and pores ( Section 4.2.5).
4.5 Electrical Properties
Electrical properties are important for biomaterials used in devices to deliver an electric current or an electrical signal. Electrically conducting metals such as platinum are used as electrodes in cardiac pacemakers and neural probes. On the other hand, electrically insulating materials such as polyurethane are used as coatings to isolate or insulate sensitive electronic devices from surrounding tissues and fluids. Whereas polymers are typically electrical insulators, several polymers have been synthesized recently which show a strong ability to conduct an electrical current. These so‐called conducting polymers have attracted interest for use in biomedical applications such as biosensors, neural probes, tissue engineering, and drug delivery.
4.5.1 Electrical Conductivity of Materials
The ability of a material to transmit an electric current is quantified by its electrical conductivity or, less commonly, by its electrical resistivity which is the inverse of the electrical conductivity. The resistivity ρ of a material is independent of its geometry but, for a wire of length l and uniform cross sectional area A , it is related to the measured electrical resistance R by the equation
(4.39) 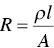
As the unit of R is ohm (Ω), the unit of ρ is ohm‐meter (Ω m), and the electrical conductivity, equal to 1/ ρ , has the unit (Ω m) −1, commonly written S/m, where S, the inverse of Ω, is the unit Siemens.
The electrical conductivity of materials covers an enormous range of ~28 orders of magnitude. Metals are good conductors, with a conductivity in the range ~10 4–0 8S/m, whereas most ceramics and polymers are insulators with conductivity in the range ~10 −20–10 −10S/m. Materials with intermediate conductivity, ~10 −6–10 4S/m, are called semiconductors ( Figure 4.16).
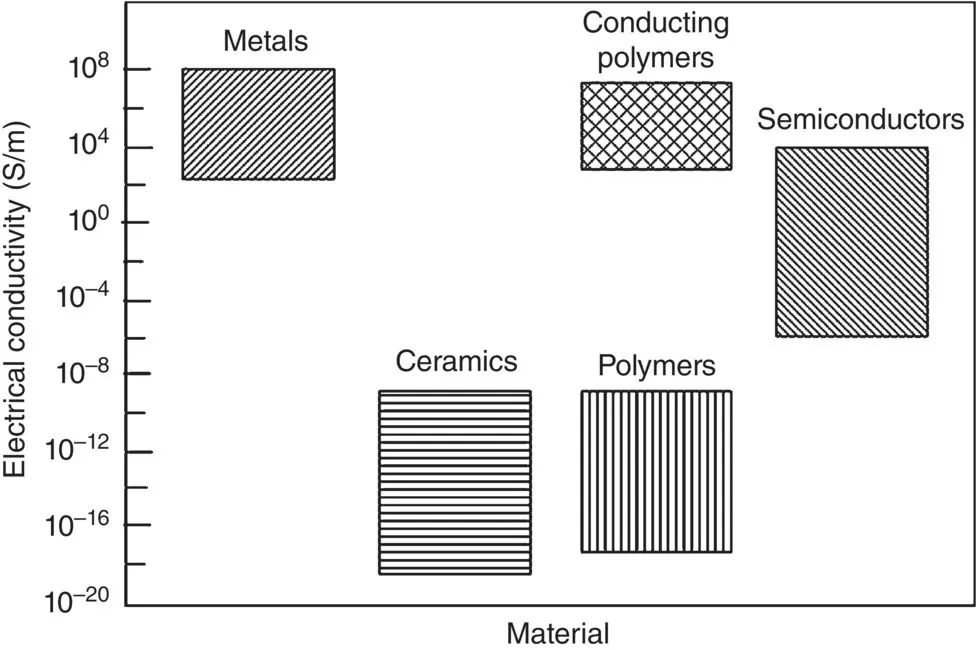
Figure 4.16 Bar chart showing the range of electrical conductivity for different types of materials at room temperature.
Transmission of an electric current results from the motion of electrically charged particles in response to forces that act on them from an externally applied electric field. The high electrical conductivity of metals arises from the ease with which the sea of nearly free electrons that surround the metal cations move through the material ( Chapter 2). Thus, metals are said to show electronic conductivity because electrons are essentially responsible for their ability to conduct an electric current. In comparison, a few ionic‐bonded ceramics can show a limited capacity to conduct an electric current, which arises from the migration of ions, referred to as ionic conductivity, plus any contribution from electronic conductivity. While the total electrical conductivity due to the electronic and ionic contributions increases with temperature, ceramics are essentially insulators at temperatures relevant to biomedical applications.
Platinum alloys (conductivity ~10 7S/m) are commonly used in cardiac pacemakers while silver alloys with a higher conductivity (~10 8S/m) find use in some implantable defibrillators. On the other hand, polyurethane (conductivity ~10 −12S/m), is often used as a coating to isolate or insulate sensitive electronic devices from surrounding tissues and fluids.
4.5.2 Electrical Conductivity of Conducting Polymers
Conducting polymers have attracted considerable interest in recent years because they can show an electrical conductivity as high as some metals ( Figure 4.16). In addition to a high electrical conductivity, conducting polymers have an attractive combination of properties that make them suitable for use as biomaterials in applications such as biosensors, neural probes, tissue engineering and drug delivery (Guimard et al. 2007). These properties include ease of synthesis, flexibility of forming into desirable shapes, which are characteristic of polymers in general, and the potential for functionalizing their surface with appropriate molecules.
Polyacetylene has shown one of the highest electrical conductivities, up to ~10 7S/m, whereas polypyrrole is the most widely studied conducting polymer. These and other conducting polymers typically show a characteristic structure composed of alternating single and double bonds in the polymer chain ( Chapter 3). While conducting polymers can show an electrical conductivity as high as some metals, the source of their electrical conductivity is not the sea of almost free electrons that surround the cations in a metal. Instead, their high conductivity arises from a combination of factors that depend on the atomic bonding and structure of the polymer.
A key factor is the alternating single and double bonds in the polymer chain backbone, allowing the π electrons of the double bond to be more easily delocalized and move more freely between the atoms of the chain (Figure a). However, this type of atomic bonding alone is not sufficient to endow the polymers with a high conductivity. Another key factor is the ability of these polymers to be doped with appropriate molecules that introduce a charge carrier into the system by removing electrons from, or adding electrons to, the polymer chain. While the mechanism is more complex, Figure 4.17illustrates a simplified explanation of the electrical conductivity (Balint et al. 2014).
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Materials for Biomedical Engineering»
Представляем Вашему вниманию похожие книги на «Materials for Biomedical Engineering» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Materials for Biomedical Engineering» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












