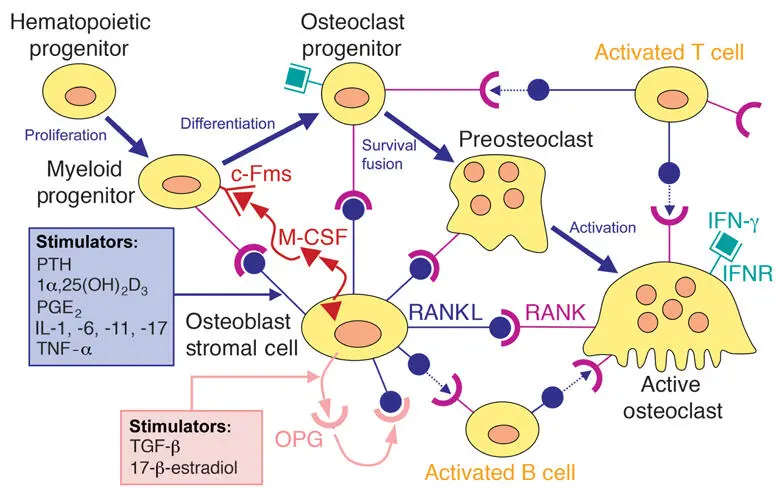
Fig 2-17RANKL/RANK/OPG system. PTH, parathyroid hormone; 1α,25[OH]2D3, 1α,25-dihydroxyvitamin D3; PGE2, prostaglandin E2; IL-1, interleukin 1; IL-6, interleukin 6; IL-11, interleukin 11; IL-17, interleukin 17; TNF-α, tumor necrosis factor α; M-CSF, macrophage colony-stimulating factor; c-Fms, M-CSF receptor; RANK, receptor activator of nuclear factor-κB; RANK-L, RANK ligand; IFN-γ, interferon γ; IFNR, IFN-γ receptor; TGF-β, transforming growth factor β; OPG, osteoprotegerin.
The discovery of this important regulatory system, which links bone biology with immune cell biology, has opened the possibility of new therapeutic strategies. Attempts using recombinant OPG were successful in preventing bone resorption and loss. However, production of significant antibody titers in a patient given OPG brought this development to an early end. Another way of blocking RANK signaling is through the use of denosumab, a fully human monoclonal antibody against RANKL. Prolia (Amgen) is used in patients with osteoporosis, whereas Xgeva (Amgen) is used for the treatment of multiple myeloma, bone metastases from solid tumors, bone giant cell tumors, and hypercalcemia of malignancy refractory to bisphosphonate therapy. Like bisphosphonates, Prolia and Xgeva can cause osteonecrosis of the jaw. These findings have led to the new term medication-related osteonecrosis of the jaw (MRONJ), which replaces the older term bisphosphonate-related osteonecrosis of the jaw (BRONJ). To help prevent MRONJ, clinicians have to ask their patients if they take antiresorptive medication. MRONJ has also been described as a complication of cancer therapies that target angiogenesis. However, this association is more controversial. Use of antiangiogenic agents as a risk factor for MRONJ among patients receiving osteoclast inhibitors for cancer is more clearly established.
Biology of Bone Regeneration
Physiologic versus reparative regeneration
Regeneration is commonly understood as replacement of vanishing or lost components in the body by tissues or organs of equally high structural organization so that structure and function are completely restored. Physiologic regeneration is distinguished from reparative regeneration. Many tissues or organ systems undergo physiologic regeneration , ie, continuous replacement of cells or tissue elements. Remodeling of cortical and trabecular bone also represents regeneration; both cells and extracellular matrix are replaced. Reparative regeneration takes place when tissues are lost because of injury or disease. Bone has the unique potential to completely restore its original architecture, but there are certain limitations. The reconstruction of the original level of tissue organization occurs sequentially and closely repeats the pattern of bone formation occurring during development and growth. Likewise, some basic conditions have to be fulfilled, such as ample blood supply and mechanical stability provided by a solid base.
Activation of bone regeneration
Any bone lesion (fracture, defect, insertion of an implant, interruption of blood supply) activates wound healing and local bone regeneration by the release and local production of growth factors and other signaling molecules, as discussed in chapter 3of this textbook. Bone is one of the richest sources of growth factors and other signaling molecules. Osteoinduction in its classic concept implies initiation of heterotopic (ectopic) bone formation; that is, bone formation at sites where bone physiologically does not normally exist. The term osteoinduction , however, is just as frequently applied if ossification is activated in contact with existing bones; that is, in the case of orthotopic bone induction. To avoid confusion, the term bone activation is preferred for orthotopic bone formation.
In heterotopic bone formation, inducible osteoprogenitor cells are found far from bone. These mesenchymal cells are abundant in subcutaneous connective tissue, skeletal muscles, and the spleen and kidney capsule. Their response to inductive stimuli, such as BMPs, is more complex than in orthotopic bone formation, and in fact mimics endochondral bone formation. 42
After subcutaneous implantation of BMPs in rats, proliferation of mesenchymal cells starts after 3 to 4 days. From days 5 to 8 onward, cartilage develops, and within 1 day it starts to mineralize. Vascular invasion and bony substitution of the calcifying cartilage follows from days 10 to 11 and onward. Intermediate cartilage differentiation seems to be mandatory if induction acts on inducible osteoprogenitor cells. Importantly, the response is always indirect bone formation.
In orthotopic bone formation, osteoprogenitor cells are found in tissues in direct proximity to bone, such as in bone marrow stroma, periosteum, endosteum, and intracortical canals. These cells respond to inductive signals with proliferation and differentiation directly into osteoblasts. Thus, in orthotopic bone induction, the inducing agents act on determined osteoprogenitor cells, and the cellular response is direct bone formation. The lag phase is short—seldom longer than 1 to 3 days—and the newly formed bone is laid down on preexisting bone surfaces.
Repair of bone defects
The repair of experimental bone defects is a good model for the study of bone regeneration. In contrast to fractures, defects are less subject to mechanical factors and to obstructions of the blood supply. Johner 43 examined the healing of bore holes with diameters of 0.1 to 1.0 mm in the rabbit tibia. Bone formation within these holes starts within a couple of days, without preceding osteoclastic resorption, and reveals a clear-cut size dependency. Holes with a diameter in the range of osteons (0.2 mm) are concentrically filled with lamellar bone. In larger holes, a scaffold of woven bone is formed first, and then lamellar bone is deposited in the newly formed intertrabecular spaces, which have a corresponding diameter of 150 to 200 µm. As in appositional growth, the matrix deposition rate of lamellar bone is restricted to a couple of microns per day, whereas woven bone rapidly bridges larger defects. After 4 weeks, both the small and the larger defects are completely filled with compact bone.
There is, however, a threshold for this rapid bridging by woven bone. This threshold lies around 1 mm for bore holes in rabbit cortical bone. 43 Experimental studies on bony ingrowth into porous acetabular components in canine hip joint arthroplasty demonstrate similar results, 44 summarized in the often-quoted phrase osteogenic jumping distance . This term indicates that bone is not able to cross gaps wider than 1 mm in one single jump. In the case of implants, the situation becomes even more difficult because bridging of the defects starts from the bony side only. This does not mean that larger holes or gaps will stay open indefinitely, but filling takes longer, and there is no doubt that bore holes of 3 to 5 mm persist for several weeks, if not months, until repair is completed.
The bone healing around dental implants has been analyzed in relation to the distance between the implant surface and the surrounding bone, 45 in relation to the implant surface characteristics, 46 , 47 and in relation to implant materials and surface topographic and chemical modifications. 48
Guided Bone Regeneration
As outlined previously, bone tissue exhibits a remarkable regenerative potential and perfectly restores its original structure and mechanical properties. However, this capacity has limitations and may even fail if certain conditions are not fulfilled. Factors that impede or even prevent bone repair include (1) failure of vascular supply; (2) mechanical instability; (3) oversized defects; and (4) competing tissues of high proliferative activity. However, several options, alone or in combination, are available to promote and to support bone formation, including:
Читать дальше













