1 ...8 9 10 12 13 14 ...25 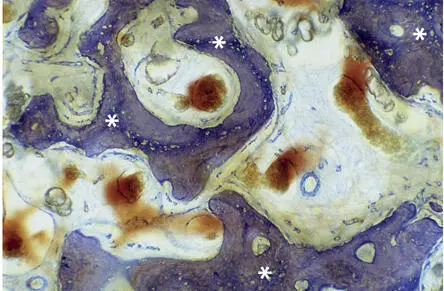
Fig 2-3Light microscopic micrograph showing reinforcement of woven bone (asterisks) by parallel-fibered bone (toluidine blue surface stain).
Mature bone consists of cortical (compact) and cancellous (trabecular or spongy) bone. Based on the orientation of the lamellae, cortical bone matrix is subdivided into different compartments. The basic structural units are the osteons or Haversian systems—longitudinally oriented cylindrical structures with vascular (Haversian) canals in the center. In secondary osteons, the wall consists of concentric lamellae, whereas primary osteons are characterized by a more primitive parallel-fibered bone matrix (see Fig 2-2). Along the periosteal and endosteal surfaces, appositional growth often results in packets of circumferential lamellae (Fig 2-4). Remnants of circumferential lamellae and of earlier generations of osteons occupy the remaining space in the form of interstitial lamellae. The osteocytes within these remnants of cortical remodeling activity are often cut off from their vascular supply and die 3 (Fig 2-5).
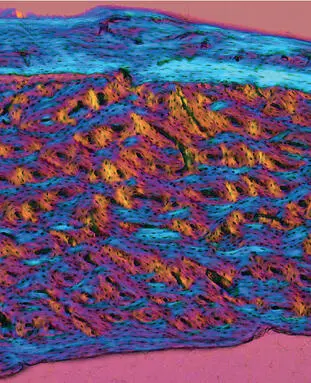
Fig 2-4Polarized light micrograph of cortical bone from a rabbit tibia. Osteons built around Haversian canals are sandwiched between circumferential lamellae at the periosteal (top) and endosteal (bottom) surfaces.

Fig 2-5Osteons are metabolic units. Staining of osteocytes demonstrates the lacunar-canalicular system. Necrotic fragment of an osteon after obliteration of the Haversian canal (asterisk) (undecalcified ground section; basic fuchsin stain).
The trabeculae of cancellous bone are also composed of bone structural units, ie, packets or walls, separated or glued together by cement lines. They also reflect local remodeling in earlier periods of growth and cancellous bone turnover. 4
Bone cells
Bone formation, maintenance, and repair are regulated by mesenchymal and bone-marrow-derived cells. Osteoblasts, osteocytes, and bone-lining cells are of mesenchymal origin, whereas osteoclasts belong to the monocyte/macrophage lineage and thus originate from bone marrow. Osteal macrophages are resident cells in bone tissue and have key functions in bone formation and remodeling. 5 Osteoblasts, bone-lining cells, and osteoclasts cover bone surfaces, whereas osteocytes are found in the interior of the bone matrix, and osteal macrophages are found in bone marrow cavities.
Osteoblasts are large cuboidal cells that form a single layer covering all periosteal or endosteal surfaces where bone formation is active. 6 They are polarized cells that secrete osteoid unidirectionally toward the bone surface. The nucleus of an osteoblast is ovoid, and its cytoplasm is filled with abundant rough endoplasmic reticulum and a prominent Golgi complex (Fig 2-6). Heterogeneity among osteoblasts seems to exist and may reflect differences between types of bone and/or anatomical sites. 6 The osteoblast is responsible for synthesis, assembly, and mineralization of the bone matrix. Osteoblasts originate from mesenchymal stem cells in bone marrow. 7 Differentiation of cells of osteoblastic lineage is controlled by multiple transcription factors at various stages of their development. Runt-related transcription factor 2 (Runx2), also known as core-binding factor α1 (Cbfa1), and osterix (Osx), downstream from Runx2, are master switches for osteoblast differentiation. 8 The expression of Runx2 is not restricted to cells of the osteogenic and chondrogenic lineage, 9 , 10 and the expression of Runx2 in fully differentiated cells suggests additional roles in osteoblast function.
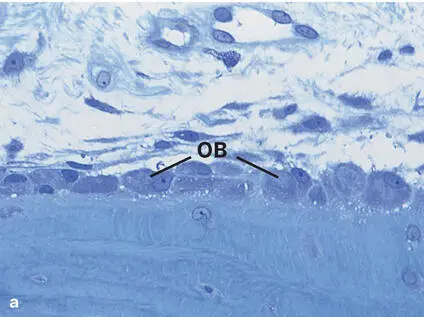
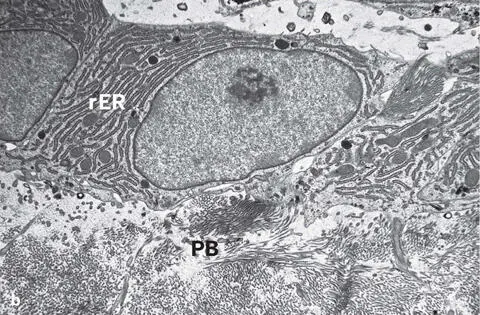
Fig 2-6 (a) Light micrograph showing a single layer of osteoblasts (OB) lining the bone matrix (toluidine blue stain). (b) Transmission electron micrograph illustrating osteoblasts with abundant rough endoplasmic reticulum (rER) lining the osteoid or prebone (PB).
Some osteoblasts become osteocytes by inversion of matrix secretion or by entrapment through neighboring osteoblasts. 11 The speed of matrix deposition may determine the number of embedded osteocytes. 12 This is exemplified in woven bone, which is formed much more quickly than any other type of bone and possesses a high number of embedded osteocytes. 13 The osteocyte is trapped in the bone matrix in a lacuna (Fig 2-7), and neighboring osteocytes are interconnected by tiny cytoplasmic processes extending through a dense canalicular system. This lacunar-canalicular system allows for diffusion of nutrients, waste products, and signaling molecules for cell communication with neighboring osteocytes, osteoblasts, bone-lining cells, osteoclasts, and macrophages. It is indispensable for osteocyte survival because diffusion of nutrients and waste products through the heavily mineralized bone matrix is almost impossible. However, the transport capacity of this system also has limitations. In mammals, the critical transport distance to keep osteocytes alive is approximately 100 µm. 14 This explains why the wall thickness of both osteons and packets in trabecular bone rarely exceeds 100 µm. Healthy osteocytes are necessary for proper functioning of bone. 15 Osteocytes are more than passive cells buried in the bone matrix; they may actively participate in bone homeostasis through their involvement in bone remodeling, ion exchange, and sensing of mechanical signals. 15 , 16 Importantly, osteocytes synthesize sclerostin, a negative regulator of bone formation, 17 and are the main source of receptor activator of nuclear factor-κB ligand (RANKL), which is required for osteoclast differentiation and activity. 18
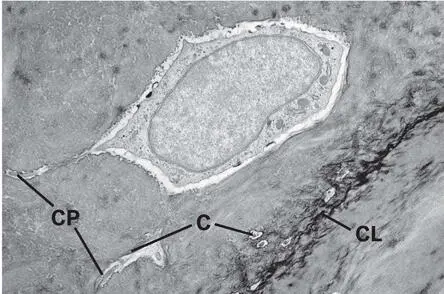
Fig 2-7Transmission electron micrograph showing an osteocyte embedded in its lacuna next to a cement line (CL). Longitudinally and transversely cut canaliculi (C) containing cytoplasmic processes (CP) are visible close to the osteocyte.
The bone-lining cell is the third cell type belonging to the osteoblast family. It is regarded as an inactive osteoblast covering the bone surface. Bone-lining cells are flat and have a reduced armamentarium of cytoplasmic organelles, which is indicative of low activity of both cell metabolism and protein synthesis. They are therefore also called inactive or resting osteoblasts . Bone-lining cells may participate in the initiation of resorption by release of osteoclast activation factors and by active contraction, which is thought to expose the bone surface for the attachment of osteoclasts. 19
The osteoclast is a large, multinucleated cell. Osteoclasts differ morphologically from other giant cells, especially from foreign-body giant cells, and are conventionally identified by their location in a resorption cavity, the Howship lacuna (Fig 2-8). Their size varies from 30 to 100 µm, and the number of nuclei varies roughly from 3 to 30. Their primary function is to degrade bone matrix, a process called bone resorption . The cytoplasm is acidophilic and often contains vacuoles (Fig 2-9a). The marginal area of the osteoclast adheres to the mineralized surface and seals off the actual resorption chamber by the so-called sealing zone (clear zone) (see Fig 2-9a). In the central part of this chamber, the cell surface is enlarged by numerous cytoplasmic undulations forming a ruffled border (Fig 2-9b). Through the enlarged cell membrane, hydrogen ions and proteolytic enzymes are released to dissolve the mineral crystals and to degrade the organic bone matrix. Osteoclasts originate from hematopoietic stem cells and develop from the self-fusion of macrophages. 20 Because of their origin, it is not surprising that many growth factors and transcription factors that are involved in hematopoietic differentiation of cells other than osteoclasts also affect osteoclast differentiation. 8 Marker proteins expressed by osteoclasts include tartrate-resistant acid phosphatase (TRAP), 21 cathepsin K, vitronectin, calcitonin, macrophage colony-stimulating factor (M-CSF), and receptor activator of nuclear factor κB (RANK).
Читать дальше


















