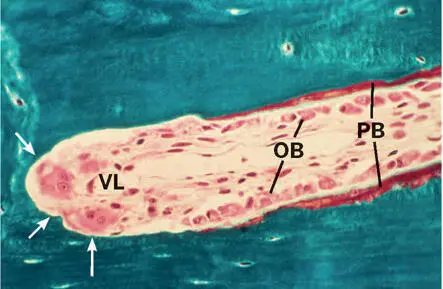
Fig 2-13Longitudinal section through the tip of an evolving secondary osteon (bone metabolizing unit) during fracture repair in a canine radius. The arrows show the osteoclastic cutting cone. VL, vascular loop; OB, osteoblasts; PB, osteoid or prebone seam (microtome section, Goldner trichrome stain).
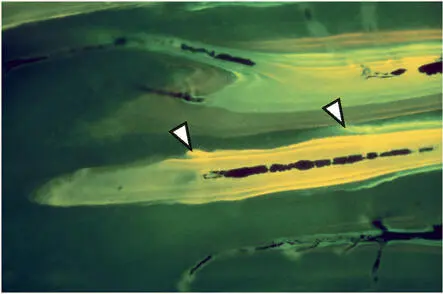
Fig 2-14Sequential polychrome labeling of a bone metabolizing unit at weekly intervals (arrowheads) to measure the daily osteoclastic resorption rate.
Cancellous bone
While the original architecture of the trabecular framework is determined by the growth pattern, modeling in the spongiosa specifically changes the architecture of cancellous bone throughout life. The trabecular network undergoes profound changes that result in a structural adaptation to the prevailing functional load, or, as often stated, “according to Wolff’s law.” 34 This adaptation enables bone to withstand a given stress with a minimum amount of material. The mechanism of functional adaptation is not fully understood, and at present Wolff’s law just offers a convenient way to accept it as a fact, without being forced to look for other explanations.
Bone remodeling improves the quality of the tissue with regard to both its mechanical and its metabolic properties. Remodeling of trabecular bone replaces discrete portions (or packets) with new lamellar bone (Fig 2-15). Formation of a new packet begins with local recruitment of osteoclasts that form a cavity on the trabecular surface. The mean depth of these cavities is around 50 µm, and it rarely exceeds 70 µm. At the end of this resorptive phase, and after a short intermission or reversal phase, osteoblasts start depositing new bone matrix during the formative phase. In analogy to the osteons, the new packet is considered a bone structural unit (BSU), and the cell populations involved in its formation are BMUs. Considering the extent of the trabecular surface in the human skeleton, the control and dynamics of cancellous bone remodeling play an important role in the pathogenesis of metabolic bone disorders, particularly in osteoporosis.
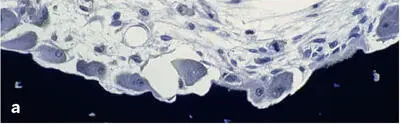
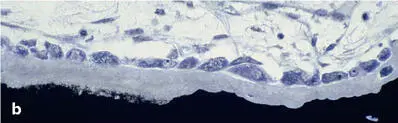
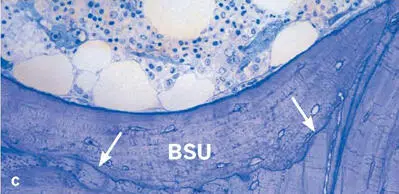
Fig 2-15Trabecular bone remodeling in a human iliac crest biopsy. (a) Resorptive phase (Von Kossa-McNeal stain). (b) Early formative phase (Von Kossa-McNeal stain). (c) Newly formed packet (bone structural unit [BSU]), clearly delineated by a reversal or cement line (arrows) (toluidine blue surface stain).
Cement lines
A cement line is a very characteristic structural entity of bone. It delineates and demarcates the interface between new and old bone. Two types of cement lines are distinguished: resting lines and reversal lines. Resting lines are smooth and strictly parallel to the lamellae. They are formed when bone formation is arrested and, after a resting period, resumes again. Reversal lines are formed during the reversal phase, ie, they constitute the matrix that is deposited directly against a bone surface previously resorbed by osteoclasts (Fig 2-16). Resting lines, which are produced by osteoblasts at sites not exposed to osteoclastic resorption, show structure and composition similar to reversal lines.
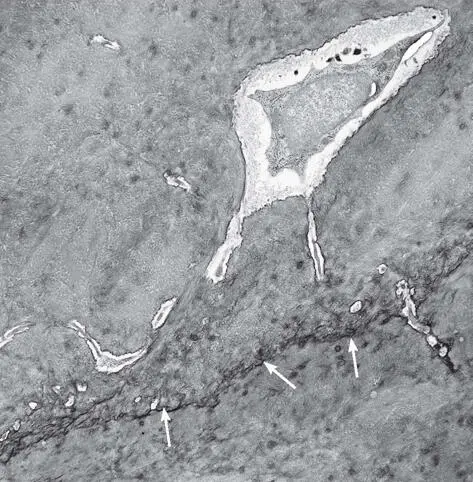
Fig 2-16Transmission electron micrograph showing a cement line (arrows) at the interface between old and new bone.
Secondary osteons are always separated from or connected to the surrounding older bone matrix by a cement line. Likewise, the new packet formed after the completion of trabecular bone remodeling is separated from the older bone matrix by a cement line. Howship lacunae left behind by the osteoclasts give the cement line a crenated appearance (see Figs 2-12c and 2-15c). The number of cement lines—both resting and reversal—indicates the intensity of matrix turnover.
Molecular aspects
Normal bone remodeling and thus bone mass maintenance depends on a delicate balance between bone formation and resorption involving a variety of cell types, including at least osteoclasts, osteoblasts, osteocytes, bone lining cells, macrophages, and blood vessels, but also cells from the immune system, which communicate with each other via signaling molecules (ie, cytokines and growth factors). The consequences of an imbalance in the expression of signaling molecules are metabolic bone disorders or diseases like Paget disease of bone, osteopetrosis, osteoporosis, arthritis, or bone loss in periodontitis. 35 , 36 Regulation of bone remodeling is under both systemic and local control. Local factors are operative in a paracrine and autocrine fashion, and osteoblasts, osteoclasts, osteocytes, and inflammatory/immune cells function as both sources and targets of signaling molecules. Numerous cytokines and growth factors have anabolic and/or catabolic effects on bone formation. 37 , 38 Among these bone-regulatory molecules are parathyroid hormone; parathyroid hormone-related peptide; calcitonin; calcitriol (the active form of vitamin D); prostaglandin E 2; growth hormone; thyroid hormone; sex steroids (estrogen and testosterone); leptin; statins; interferon γ; tumor necrosis factor α (TNF-α); transforming growth factor α (TGF-α); TGF-β; bone morphogenetic proteins (BMPs); fibroblast growth factor (FGF); insulin-like growth factor 1 (IGF-1); platelet-derived growth factors (PDGF); interleukins (IL) 1, 6, 11, and 17; and sclerostin.
A breakthrough in bone and immunology research was the detection of the RANKL/RANK/OPG system, which was first identified in the late 1990s as a pivotal regulator of bone remodeling. Bone resorption is regulated by this system, consisting of RANK and its ligand RANKL (which are members of the TNF ligand and receptor families) and osteoprotegerin (OPG). RANKL is expressed mainly by osteocytes, but also by bone marrow stromal cells, osteoblasts, and certain fibroblasts, whereas RANK is expressed by osteoclast precursors and mature osteoclasts. The binding of RANK to RANKL induces osteoclast differentiation and activity and regulates osteoclast survival. OPG, however, is produced by osteoblasts, bone marrow stromal cells, and other cell types and is a soluble decoy receptor for RANKL that competes for this binding. Thus, OPG is a natural inhibitor of osteoclast differentiation and activation.
Any interference with this system can shift the balance between bone apposition and resorption. The expression of M-CSF plays an essential role in this regulatory system. Furthermore, it has been shown that a number of proinflammatory cytokines and growth factors, in particular IL-1 and TNF-α, regulate the expression of RANKL and OPG (Fig 2-17). The immune system modifies the balance between bone formation and resorption in a complex process involving T and B lymphocytes, dendritic cells, and cytokines. By the expression of RANKL on B cells, T cells, and marrow stromal cells, and the expression of RANK on osteoclast precursors, mature osteoclasts, T lymphocytes, B lymphocytes, and dendritic cells, these cells can directly influence bone resorption. 39 – 41
Читать дальше


















