Osteoinduction by growth factors
Osteoconduction by autogenous bone grafts or substitutes
Transfer of stem cells or progenitor cells that differentiate into osteoblasts
Distraction osteogenesis
GBR using barrier membranes
GBR, usually in combination with a bone grafting material, is the most widely used method of augmenting bone in routine dental practice. The use of barrier membranes for bone regeneration was adapted from studies on periodontal regeneration 49 , 50 and started in the mid 1980s with preclinical experiments. 51 – 53
The GBR principle
The key principle of GBR is to keep undesirable cells from nonosteogenic tissues from interfering with bone regeneration. To this end, a physical barrier membrane is placed between the region to be augmented with new bone and the adjacent soft tissue. Because bone is a relatively slow-growing tissue, both fibroblasts and epithelial cells have the opportunity to occupy available space more efficiently during wound healing and to build up a soft connective tissue much faster than bone is able to grow. If the occlusive barrier function lasts long enough and if the barrier membrane is not exposed to the oral cavity, optimal conditions exist for the ingrowth of blood vessels from the resident bone, allowing stem cells and osteoprogenitor cells to differentiate into osteoblasts, which produce the bone matrix. In other words, the barrier membrane creates a secluded space that allows bone to use its great, natural healing capacity in an undisturbed or protected manner.
It is noteworthy that the theory of total cell occlusion has been challenged by the awareness that nutrient transfer across the membrane may be important for successful bone regeneration. 54 , 55 Indeed, macroporous membranes were found to be more predictable GBR and for guided tissue regeneration (GTR) procedures and more conducive to uncomplicated clinical management than occlusive membranes. 56 , 57 Very recently, the function of a membrane as a mere tissue-separating barrier has been further questioned. There is some evidence suggesting that certain membranes have functions beyond the barrier role. 58 These emerging data suggest that the membrane contributes actively to the regeneration of underlying bone defects. It would not be surprising if the specific macrophage response to a biomaterial is involved in this contributing effect.
Biomaterials used for GBR
Barrier membranes
In practice, the barrier membrane is placed in direct contact with the outer surface of the bone surrounding the defect, and the mucoperiosteal flap is then repositioned and sutured. Clinicians have access to a wide range of membrane materials for GBR. To select the material best suited for a specific clinical application, it is necessary to understand the basic requirements for membrane materials used in these indications. These basic characteristics include:
Biocompatibility
Cell occlusion (occlusivity, occlusiveness)
Space-making and space-maintaining ability
Tissue integration
Degradability
Clinical handling
Susceptibility to complications
While in the pioneering phase of GBR in the late 1980s and 1990s, the nonresorbable expanded polytetrafluoroethylene (ePTFE) membrane prevailed, resorbable membranes were evaluated later. Both nonresorbable and bioresorbable membranes have inherent advantages and disadvantages.
Nonresorbable membranes
The most widely used nonresorbable membrane type is made of PTFE. It was originally developed in the late 1960s, and marketing began in 1971. PTFE is hydrophobic in nature and biologically inert, which makes this biomaterial nonresorbable. Thus, the major advantage is its excellent barrier function, whereas the major disadvantage is the need for a second surgery to remove the membrane. The ePTFE membrane is produced by exposing PTFE to high tensile strain, resulting in expansion and formation of a porous microstructure. The most commonly used ePTFE membrane has a two-part design with a dense portion and a less dense portion. The inner (central) dense portion, located over the defect space, has a pore size of less than 8 µm to allow fluid exchange while preventing infiltration of cells (Fig 2-18a). In contrast, the microstructure of the outer portion, which is in contact with the bone at the defect margin, is less dense; it has pores 20 to 25 µm wide and a surface structure that encourages blood clot adhesion and soft connective tissue attachment to and invasion into the membrane, ultimately leading to tissue integration (Fig 2-18b). In dentistry, ePTFE membranes became the standard for guided tissue regeneration (GTR) and GBR procedures during the development phase of both techniques in the 1980s and early 1990s. 59
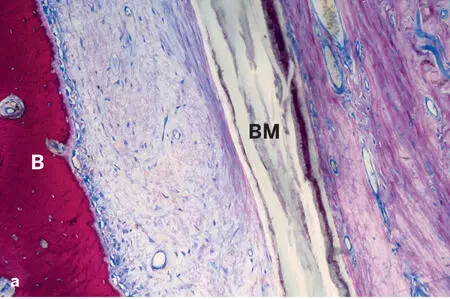
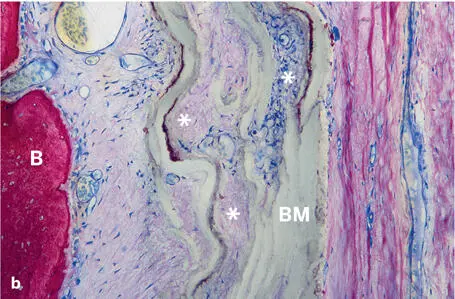
Fig 2-18Soft and hard tissue compartments adjacent to an ePTFE barrier membrane (BM) (ground sections; toluidine blue and basic fuchsin surface stain). (a) The dense portion of the barrier membrane separates an outer, mucosal compartment from an inner, soft connective tissue compartment that is mainly accessible from the bone (B) compartment. There are no signs of a foreign-body reaction. (b) The porous part of the barrier membrane (asterisks) allows ingrowth of blood vessels and cells into the gaps in the membrane.
High-density PTFE (dPTFE) is different from ePTFE in that it possesses pores in the submicron scale (0.2 µm). Bacterial adhesion and infiltration is eliminated because of its high density and small pore size. Primary soft tissue closure is not required, allowing tissue healing with a barrier membrane being exposed to the oral cavity.
To overcome the problem of membrane collapse, the PTFE membrane can be mechanically stabilized with titanium, which is known as a titanium-reinforced PTFE . Alternatively, membranes made of metal meshes consisting of titanium or titanium alloy and cobalt-chromium alloy may be used. 60
As already mentioned, one big disadvantage of nonresorbable membranes is the need for their removal with an additional surgical intervention. Furthermore, complications associated with ePTFE membranes include difficult handling because of their hydrophobic nature, membrane collapse, and membrane exposure, often leading to infection and compromised regenerative outcome. These complications were the stimulus for the testing of another generation of biomaterials, the bioresorbable membranes.
Bioresorbable membranes
As the name indicates, bioresorbable or biodegradable membranes are broken down in the body and disappear over time. Thus, they have the advantage of eliminating the need for an additional surgery to remove the biomaterial. Two main categories of bioresorbable membranes exist: (1) synthetic polymers, and (2) polymers derived from various animal sources. Each membrane has distinct physicochemical properties and biologic effects. The degradation process has an important influence on the outcome, since (1) if degradation occurs too quickly, the biomaterial may not have a chance to fulfill its function as a barrier, and (2) products of degradation may contribute to unfavorable tissue responses, including foreign-body reactions, which may prevent tissue integration and bone formation and even result in bone resorption. 61 Most clinically used bioresorbable membranes are made of processed collagen or aliphatic polyesters.
Synthetic membranes
Читать дальше














