 The green color of many plant leaves is due to the magnesium-containing compound chlorophyll.
The green color of many plant leaves is due to the magnesium-containing compound chlorophyll.
Chapter 2
Seems So Basic: Water Chemistry and pH
IN THIS CHAPTER
 Understanding the roles and properties of water
Understanding the roles and properties of water
 Exploring the differences between acids and bases
Exploring the differences between acids and bases
 Controlling pH with buffers
Controlling pH with buffers
Water is one of the most important substances on Earth. People swim, bathe, boat, and fish in it. It carries waste from people’s homes and is used in the generation of electrical power. Humans drink it in a variety of forms: pure water, soft drinks, tea, coffee, beer, and so on. Water, in one form or another, moderates the temperature of the Earth and of the human body.
In the area of biochemistry, water is also one of the lead actors. The human body is about 70 percent water. Water plays a role in the transport of material to and from cells. And many, many aqueous solutions take part in the biochemical reactions in the body.
In this chapter, we examine the water molecule’s structure and properties. We explain how water behaves as a solvent. We also look at the properties of acids and bases, and the equilibria that they may undergo. Finally, we discuss the pH scale and buffers, including the infamous Henderson-Hasselbalch equation. So sit back, grab a glass of water (or your favorite water-based beverage), and dive in!
Water is essential to life; in fact, human beings are essentially big sacks of water. Water accounts for 60 to 95 percent of living human cells, and 55 percent of the water in the human body is in intracellular fluids. The remaining 45 percent (extracellular) is divided among the following:
Plasma (8 percent)
Interstitial (between cells) and lymph (22 percent)
Connective tissue, cartilage, and bone (15 percent)
Water also is necessary as a solvent for the multitude of biochemical reactions that occur in the body:
Water acts as a transport medium across membranes, carrying substances into and out of cells.
Water helps maintain body temperature.
Water acts as a solvent (carrying dissolved chemicals) in the digestive and waste excretion systems.
Healthy humans have an intake/loss of about 2 liters of water per day. The intake is about 45 percent from liquids and 40 percent from food, with the remainder coming from the oxidation of food. The loss is about 50 percent from urine and 5 percent from feces, with the remainder leaving through evaporation from the skin and lungs. A water balance must be maintained within the body. If the water loss significantly exceeds the intake, the body experiences dehydration. If the intake significantly exceeds the water loss, water builds up in the body and causes edema (fluid retention in tissues).
In the following sections, we touch on the basic properties of this must-have liquid, as well as its most important biochemical function.
Let’s get wet! The physical properties of water
The medium in which biological systems operate is water, and the physical properties of water influence the biological systems. Therefore, it’s important to review some water properties from general chemistry.
Water is a polar molecule
Water is a bent molecule, not linear (see Figure 2-1). The hydrogen atoms have a partially positive charge 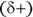 ; the oxygen atom has a partially negative charge
; the oxygen atom has a partially negative charge 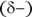 . This charge distribution is due to the electronegativity difference between hydrogen and oxygen atoms (the attraction that an atom has for a bonding pair of electrons). The water molecule in Figure 2-1 is shown in its bent shape with a bond angle of about 105 degrees.
. This charge distribution is due to the electronegativity difference between hydrogen and oxygen atoms (the attraction that an atom has for a bonding pair of electrons). The water molecule in Figure 2-1 is shown in its bent shape with a bond angle of about 105 degrees.
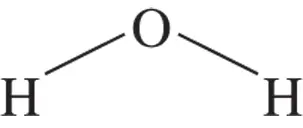
FIGURE 2-1:Structure of a water molecule.
Water has strong intermolecular forces
Normally, partial charges such as those found in a water molecule result in an intermolecular force known as a dipole-dipole force, in which the positive end of one molecule attracts the negative end of another molecule. The very high electronegativity of oxygen combined with the fact that a hydrogen atom has only one electron results in a charge difference significantly greater than you’d normally expect. This charge difference leads to stronger-than-expected intermolecular forces (stronger than the dipole-dipole forces), and these forces have a special name: hydrogen bonds.
 The term hydrogen bond doesn’t refer to an actual bond to a hydrogen atom but to the overall interaction of a hydrogen atom bonded to either oxygen, nitrogen, or fluorine atoms with an oxygen, nitrogen, or fluorine on another molecule (intermolecular) or the same molecule (intramolecular). Hence the term intermolecular force. (Note that although hydrogen bonds occur when hydrogen bonds to fluorine, you don’t normally find such combinations in biological systems.)
The term hydrogen bond doesn’t refer to an actual bond to a hydrogen atom but to the overall interaction of a hydrogen atom bonded to either oxygen, nitrogen, or fluorine atoms with an oxygen, nitrogen, or fluorine on another molecule (intermolecular) or the same molecule (intramolecular). Hence the term intermolecular force. (Note that although hydrogen bonds occur when hydrogen bonds to fluorine, you don’t normally find such combinations in biological systems.)
Hydrogen bonds in oxygen- and nitrogen-containing molecules are very important in biochemistry because they influence reactions between such molecules and the structures of these biological molecules. The interaction between water and other molecules in which there may be an opportunity for hydrogen bonding explains such properties as solubility in water and reactions that occur with water as a solvent (more on that in a minute).
 One environmentally important consequence of hydrogen bonding is that, upon freezing, water molecules are held in a solid form that’s less dense than the liquid form. The hydrogen bonds lock the water molecules into a crystalline lattice that contains large holes, which decreases the density of the ice. The less-dense ice — whether in the form of an ice cube or an iceberg — floats on liquid water. In nearly all other cases where a solid interacts with water, the reverse is true: The solid sinks in the liquid. So why is the buoyancy of ice important? Ask ice fishermen! The layer of ice that forms on the surface of cold bodies of water insulates the liquid from the cold air, protecting the organisms that live under the ice.
One environmentally important consequence of hydrogen bonding is that, upon freezing, water molecules are held in a solid form that’s less dense than the liquid form. The hydrogen bonds lock the water molecules into a crystalline lattice that contains large holes, which decreases the density of the ice. The less-dense ice — whether in the form of an ice cube or an iceberg — floats on liquid water. In nearly all other cases where a solid interacts with water, the reverse is true: The solid sinks in the liquid. So why is the buoyancy of ice important? Ask ice fishermen! The layer of ice that forms on the surface of cold bodies of water insulates the liquid from the cold air, protecting the organisms that live under the ice.
Water has a high specific heat
Specific heat is the amount of heat required to increase the temperature of a gram of water by 1 degree Celsius. The high specific heat of water means that changing the temperature of water isn’t easy. Water also has a high heat of vaporization, the quantity of heat required at a specified temperature to convert a specified mass of liquid into vapor . Humans can rid their bodies of a great deal of heat when their sweat evaporates from their skin, making sweat a very effective cooling method. We’re sure you’ll notice this cooling effect during your biochem exams.
Читать дальше

 The green color of many plant leaves is due to the magnesium-containing compound chlorophyll.
The green color of many plant leaves is due to the magnesium-containing compound chlorophyll. Understanding the roles and properties of water
Understanding the roles and properties of water ; the oxygen atom has a partially negative charge
; the oxygen atom has a partially negative charge 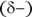 . This charge distribution is due to the electronegativity difference between hydrogen and oxygen atoms (the attraction that an atom has for a bonding pair of electrons). The water molecule in Figure 2-1 is shown in its bent shape with a bond angle of about 105 degrees.
. This charge distribution is due to the electronegativity difference between hydrogen and oxygen atoms (the attraction that an atom has for a bonding pair of electrons). The water molecule in Figure 2-1 is shown in its bent shape with a bond angle of about 105 degrees.
 The term hydrogen bond doesn’t refer to an actual bond to a hydrogen atom but to the overall interaction of a hydrogen atom bonded to either oxygen, nitrogen, or fluorine atoms with an oxygen, nitrogen, or fluorine on another molecule (intermolecular) or the same molecule (intramolecular). Hence the term intermolecular force. (Note that although hydrogen bonds occur when hydrogen bonds to fluorine, you don’t normally find such combinations in biological systems.)
The term hydrogen bond doesn’t refer to an actual bond to a hydrogen atom but to the overall interaction of a hydrogen atom bonded to either oxygen, nitrogen, or fluorine atoms with an oxygen, nitrogen, or fluorine on another molecule (intermolecular) or the same molecule (intramolecular). Hence the term intermolecular force. (Note that although hydrogen bonds occur when hydrogen bonds to fluorine, you don’t normally find such combinations in biological systems.) One environmentally important consequence of hydrogen bonding is that, upon freezing, water molecules are held in a solid form that’s less dense than the liquid form. The hydrogen bonds lock the water molecules into a crystalline lattice that contains large holes, which decreases the density of the ice. The less-dense ice — whether in the form of an ice cube or an iceberg — floats on liquid water. In nearly all other cases where a solid interacts with water, the reverse is true: The solid sinks in the liquid. So why is the buoyancy of ice important? Ask ice fishermen! The layer of ice that forms on the surface of cold bodies of water insulates the liquid from the cold air, protecting the organisms that live under the ice.
One environmentally important consequence of hydrogen bonding is that, upon freezing, water molecules are held in a solid form that’s less dense than the liquid form. The hydrogen bonds lock the water molecules into a crystalline lattice that contains large holes, which decreases the density of the ice. The less-dense ice — whether in the form of an ice cube or an iceberg — floats on liquid water. In nearly all other cases where a solid interacts with water, the reverse is true: The solid sinks in the liquid. So why is the buoyancy of ice important? Ask ice fishermen! The layer of ice that forms on the surface of cold bodies of water insulates the liquid from the cold air, protecting the organisms that live under the ice.










