John O'Brien - Earth Materials
Здесь есть возможность читать онлайн «John O'Brien - Earth Materials» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Earth Materials
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Earth Materials: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Earth Materials»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Earth Materials,
Earth Materials,
Earth Materials — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Earth Materials», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Table 2.5 Relationship between radius ratio, coordination number, and coordination polyhedra.
| Radius ratio (Rc/Ra) | Coordination number | Coordination type | Coordination polyhedron |
|---|---|---|---|
| <0.155 | 2 | Linear | Line |
| 0.155–0.225 | 3 | Triangular | Triangle |
| 0.225–0.414 | 4 | Tetrahedral | Tetrahedron |
| 0.414–0.732 | 6 | Octahedral | Octahedron |
| 0.732–1.00 | 8 | Cubic | Cube |
| >1.00 | 12 | Cubic or hexagonal closest packed | Cubeoctahedron complex |
The general relationship between radius ratio, coordination number and the type of coordination polyhedron that results is summarized in Table 2.5. For radius ratios less than 0.155, the coordination number is 2 and the “polyhedron” is a line. The appearance of these coordination polyhedra is summarized in Figure 2.19.
When predicting coordination number using radius ratios, several caveats must be kept in mind.
1 The ionic radius and coordination number are not independent. As illustrated by Table 2.6, effective ionic radius increases as coordination number increases.
2 Since bonds are never truly ionic, models based on spheres in contact are only approximations. As bonds become more covalent and more highly polarized, radius ratios become increasing less effective in predicting coordination numbers.
3 Radius ratios do not successfully predict coordination numbers for metallically bonded substances.
The great value of the concept of coordination polyhedra is that it yields insights into the fundamental patterns in which atoms bond during the formation of crystalline materials. These patterns most commonly involve threefold (triangular), fourfold (tetrahedral), sixfold (octahedral), eightfold (cubic) and, to a lesser extent, 12‐fold coordination polyhedra or small variations of such basic patterns. Other coordination numbers and polyhedron types exist, but are rare in inorganic Earth materials.
Another advantage of using spherical ions to model coordination polyhedra is that it allows one to calculate the size or volume of the resulting polyhedron. In a coordination polyhedron of anions, the cation–anion distance is determined by the radius sum (R ∑ ). The radius sum is simply the sum of the radii of the two ions (Rc + Ra); that is, the distance between their respective centers. Once this is known, the size of any polyhedron can be calculated using the principles of geometry. Such calculations are beyond the scope of this book but are discussed in Wenk and Bulakh (2016) and Klein and Dutrow (2007).
2.4.2 Electrostatic valency
An important concept related to the formation of coordination polyhedra is electrostatic valency (EV). In a stable coordination structure, the total strength of all the bonds that reach a cation from all neighboring anions is equal to the charge on the cation. This is another way of saying that the positive charge on the cation is neutralized by the electrostatic component of the bonds between it and its nearest neighbor anions. Similarly, every anion in the structure is surrounded by some number of nearest neighbor cations to which it is bonded, and the negative charges on each anion are neutralized by the electrostatic component of the bonds between it and its nearest neighbor cations. For a cation of charge Z bonded to a number of nearest neighbor anions (CN), the electrostatic valency of each bond is given by the charge of the cation divided by the number of nearest neighbors to which it is coordinated:

For example, in the case of the silica tetrahedron (SiO 4) −4each Si +4cation is coordinated with four O −2anions ( Figure 2.20). The electrostatic valency of each bond is given by EV = Z/CN = +4/4 = +1. What this means is that each bond between the coordinating silicon ion (Si +4) and the coordinated oxygen ions (O −2) balances a charge of +1. Another way to look at this is to say that each bond involves an electrostatic attraction between ions of opposite charge of one charge unit. Since there are four Si–O bonds, each balancing a charge of +1, the +4 charge on the silicon ion is fully neutralized by the four nearest neighbor anions to which it is bonded. However, although the +4 charge on the coordinating silicon ion is fully satisfied, the −2 charge on each of the coordinated ions is not. Since each has a −2 charge, a single bond involving an electrostatic attraction of one charge unit neutralizes only half their charge. They must attract and bond to one or more additional cations, with an additional total electrostatic valency of one, in order to have their charges effectively neutralized. So it is that during mineral growth, cations attract anions and anions attract additional cations of the appropriate charge and radius which in turn attract additional anions of the appropriate charge and radius as the mineral grows. In this manner minerals retain their essential geometric patterns and their ions are neutralized as the mineral grows. In the following section we will introduce the major mineral groups and see how their crystal chemistry forms the basis of the mineral classification.
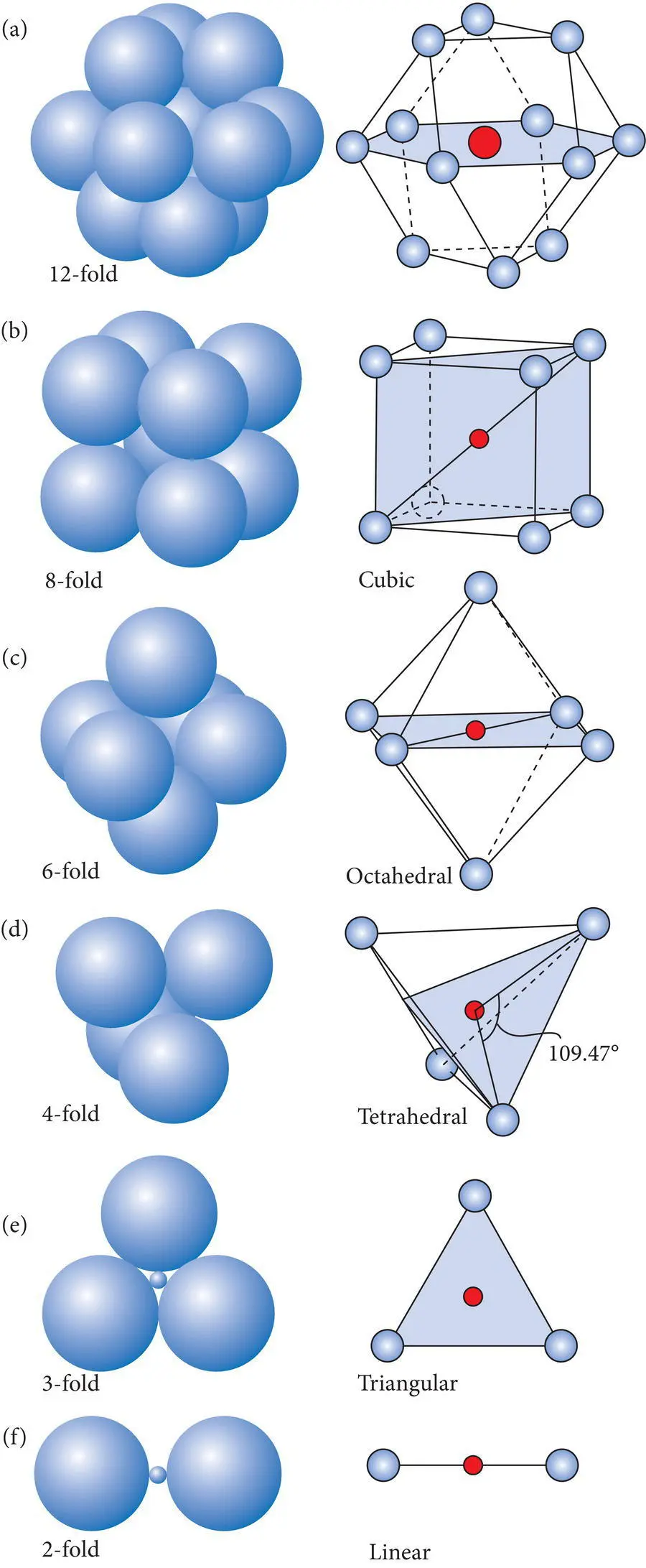
Figure 2.19 Common coordination polyhedra: (a) cubic closest packing, (b) cubic, (c) octahedral, (d) tetrahedral, (e) triangular, (f) linear.
Source : Wenk and Bulakh (2004). © Cambridge University Press.
Table 2.6 Variations in ionic radius (in angstroms) with coordination number (CN) for some common cations.
| Ion | CN = 4 | CN = 6 | CN = 8 |
|---|---|---|---|
| Na +1 | 0.99 | 1.02 | 1.18 |
| K +1 | 1.38 | 1.51 | |
| Rb +1 | 1.52 | 1.61 | |
| Cs +1 | 1.67 | 1.74 | |
| Mg +2 | 0.57 | 0.72 | |
| Al +3 | 0.39 | 0.48 | |
| Si +4 | 0.26 | 0.40 | |
| P +5 | 0.17 | 0.38 | |
| S +6 | 0.12 | 0.29 |
2.5 THE CHEMICAL CLASSIFICATION OF MINERALS
The formation and growth of most minerals can be modeled by the attractive forces between cations and anions, the formation of coordination polyhedra with unsatisfied negative charges and the attraction of additional ions to build additional coordination polyhedra ad infinitum , until the conditions for growth cease to exist. It is useful to visualize minerals in terms of major anions and anion groups and/or radicals bonded to various cations that effectively neutralize their charge during the formation and growth of minerals. One common way to group or classify minerals is to do so in terms of the major anion group in the mineral structure. Those that contain (SiO 4) −4silica tetrahedra, discussed in the previous section, are silicate minerals, by far the most common minerals in Earth's crust and upper mantle. Those that do not contain silica tetrahedra are nonsilicate minerals and are further subdivided on the basis of their major anions. Table 2.7summarizes the common mineral groups according to this classification system. These groups are discussed in more detail in Chapter 5.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Earth Materials»
Представляем Вашему вниманию похожие книги на «Earth Materials» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Earth Materials» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












