John O'Brien - Earth Materials
Здесь есть возможность читать онлайн «John O'Brien - Earth Materials» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Earth Materials
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Earth Materials: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Earth Materials»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Earth Materials,
Earth Materials,
Earth Materials — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Earth Materials», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
6 Using the periodic table of the elements ( Table 2.3), calculate the electronegativity difference and then predict the bond type and properties for each of the following minerals:Native platinum (Pt)Periclase (MgO)Sylvite (KCl)Pyrite (FeS2)Sphalerite (ZnS).
7 Explain the concepts of radius ratio and electrostatic valency and use them to explain why silica tetrahedra tend to link by sharing oxygen ions. Then describe and explain the basic differences between nesosilicate, sorosilicate, cyclosilicate, nesosilicate, phyllosilicate and tectosilicate structures.
REFERENCES
1 Klein, C. and Dutrow, B. (2007). Manual of Mineral Science (Manual of Mineralogy), 23e. New York: Wiley 704 pp.
2 Pauling, L. (1929). The principles determining the structure of complex ionic structures. Journal American Chemical Society 51: 1010–1026.
3 Railsback, L.B. (2003). An earth scientist's periodic table of the elements and their ions. Geology 31: 737–740.
4 Wenk, H.R. and Bulakh, A. (2004). Minerals: Their Constitution and Origin, 3e. Cambridge, UK: Cambridge University Press 646 pp.
5 Wenk, H.R. and Bulakh, A. (2016). Minerals: Their Constitution and Origin, 3e. Cambridge, UK: Cambridge University Press 621 pp.
Chapter 3 Atomic substitution, phase diagrams, and isotopes
1 3.1 Atomic (ionic) substitutio
2 3.2 Phase stability (equilibrium) diagrams
3 3.3 Isotopes
3.1 ATOMIC (IONIC) SUBSTITUTION
Minerals are composed of atoms or ions that occupy structural sites in a crystal structure ( Chapter 2). Different ions can occupy the same structural site if (1) they have similar size, (2) have similar charge, and (3) are available in the environment in which the mineral is forming. This process of one ion replacing another ion is called ionic substitution. In mineral formulas, ions that commonly substitute for one another are generally placed within a single set of parentheses. In the olivine group, iron and magnesium can freely substitute for one another in the sixfold, octahedral site. As a result, the formula for olivine is commonly written as (Mg,Fe) 2SiO 4.
Substitution is favored for ions of similar ionic radius. In general, cation substitution at surface temperatures and pressures is limited when the larger cation radii exceed the smaller by 10–15% and becomes negligible for differences greater than 30%. Such ions are “too big” or “too small” to easily substitute for one another ( Figure 3.1a), while ions of similar size are “just right.” Substitution of ions of significantly different radii distorts coordination polyhedra and decreases the stability of crystals. However, at higher temperatures, where the crystal structure is expanded, ions with larger differences in radius may more easily substitute for one another.
Substitution is favored for ions of similar charge. Where substitutions occur in only one coordination site, substitution is largely limited to ions with the same charge ( Figure 3.1b). This enables the mineral to remain electrically neutral, which increases its stability. However, where substitution can occur in multiple coordination sites, ions of different charge may substitute for one another in one site so long as this charge difference is balanced by a second substitution of ions of different charge in a second coordination site.

Figure 3.1 Criteria for substitution are (a) similar size, (b) similar charge and (not shown) availability.
Substitution is favored for ions that are widely available in the environment in which the mineral is growing ( Figure 3.1). As minerals grow, coordination sites will preferentially select ions with the appropriate radii and charge that are available in the vicinity of the growing crystal. The ions that occupy a coordination site in a mineral provide vital clues to the chemical composition of the system and environmental conditions under which crystallization occurred.
3.1.1 Simple ionic substitution
Simple, complete substitutionexists when two or more ions of similar radii and the same charge may substitute for one another in a coordination site in any proportions. In such cases, it is convenient to define end membersor componentsthat have only one type of ion in the structural site in question. The olivine group illustrates complete substitution. In the olivine group, (Mg,Fe) 2SiO 4, Mg +2(radius = 0.66 Å) and Fe +2(radius = 0.74 Å) can substitute for one another in the octahedral site in any proportion. The two end members are the pure magnesium silicate component called forsterite[(Mg) 2SiO 4] and the pure iron silicate component called fayalite[(Fe) 2SiO 4]. Since these two end members can substitute for one another in any proportion in olivine, a complete solid solution seriesexists between them. As a result, the composition of any olivine can be expressed in terms of the proportions of forsterite (Fo) and/or fayalite (Fa). Simple two‐component, complete solid solution series are easily represented by a number line called a tie linebetween the two end members ( Figure 3.2).
Compositions of any olivine can be represented in a number of different ways. For example, pure magnesium olivine can be represented by (1) a formula (Mg 2SiO 4), (2) a name (forsterite), (3) its position on the tie line (far right) or (4) the proportion of either end member (Fo 100or Fa 0). Similarly, pure iron olivine can be represented by a formula (Fe 2SiO 4), a name (fayalite), its position on the tie line (far left) or the proportion of either end member (Fo 0or Fa 100). Any composition in the olivine complete solid solution series can be similarly represented. For example, the composition of an olivine with equal amounts of the two end member components can be represented by the formula [(Mg 0.5,Fe 0.5) 2SiO 4], its position on the tie line (halfway between the ends), or the proportions of either end member (Fo 50or Fa 50). Typically the forsterite component is used (e.g., Fo 50) and the fayalite (Fa) component (100 – Fo) is implied.
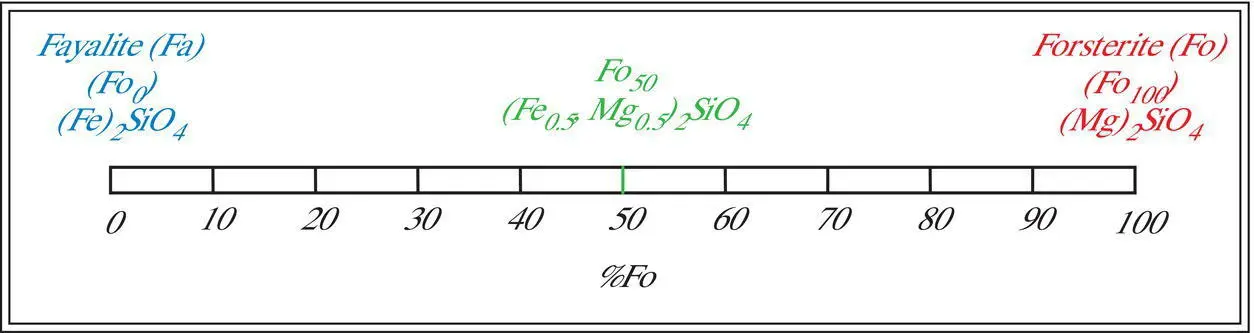
Figure 3.2 Olivine complete substitution solid solution series.
In cases where three ions substitute freely for one another in the same coordination site, it is convenient to define three end member components. Each of these end member components contains only one of the three ions in the structural site in which substitution occurs. For example, ferrous iron (Fe +2), magnesium (Mg +2), and manganese (Mn +2) can all substitute for one another in any proportions in the cation site of rhombohedral carbonates. The general formula for such carbonate minerals can be written as (Fe,Mg,Mn)CO 3.The three end member components are the “pure” minerals siderite (FeCO 3), magnesite (MgCO 3), and rhodochrosite (MnCO 3). On a three‐component diagram, the three pure end member components are plotted at the three apices of a triangle ( Figure 3.3).
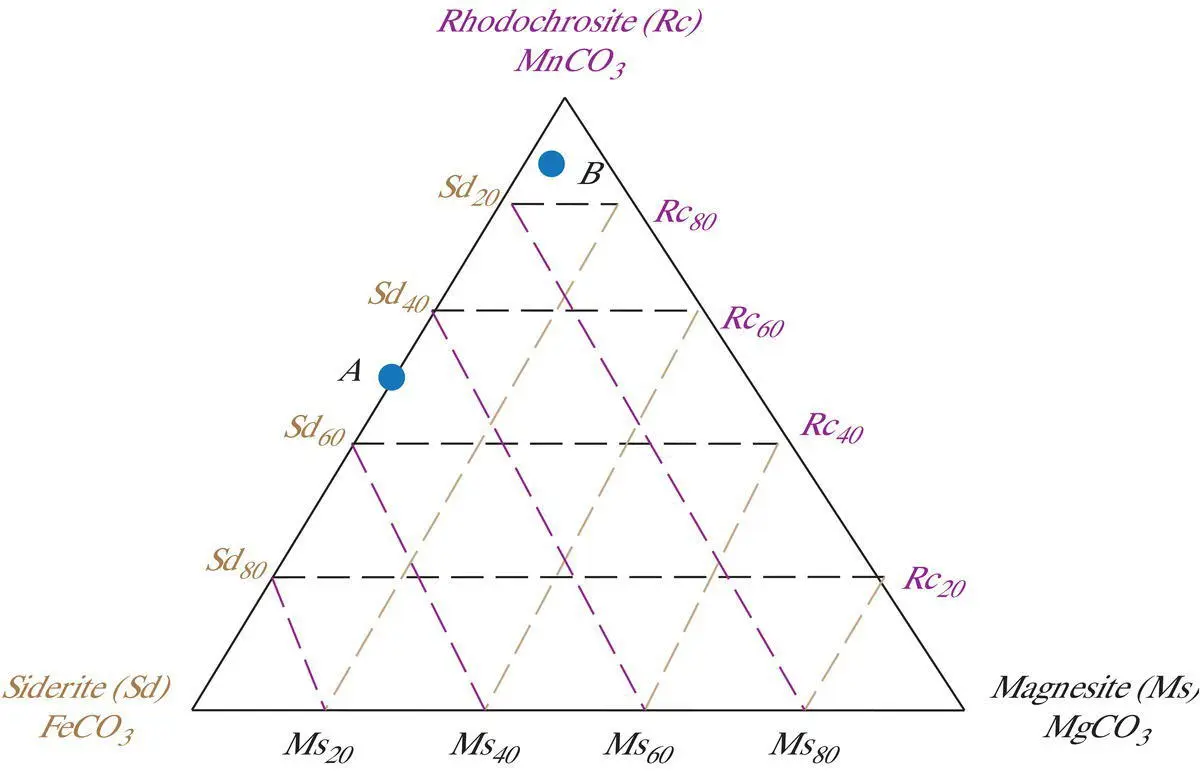
Figure 3.3 Compositions of carbonate minerals expressed in terms of the proportions of iron, magnesium, and manganese; that is of the three components: siderite (Sd), magnesite (Ms), and rhodochrosite (Rc) plotted on a ternary diagram.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Earth Materials»
Представляем Вашему вниманию похожие книги на «Earth Materials» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Earth Materials» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












