John O'Brien - Earth Materials
Здесь есть возможность читать онлайн «John O'Brien - Earth Materials» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Earth Materials
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Earth Materials: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Earth Materials»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Earth Materials,
Earth Materials,
Earth Materials — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Earth Materials», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Points on the apices of the triangle represent “pure” carbonate minerals with only one end member component. Percentages of any component decrease systematically from 100% at the apex toward the opposite side of the triangle where its percentage is zero. Each side of the triangle is a tie line connecting two end members. Points on the sides represent carbonate solid solutions between two end member components. Point A on Figure 3.3lies on the side opposite the magnesite apex and so contains no magnesium. Because it lies halfway between rhodochrosite and siderite, its composition may be written as Rc 50Sd 50or as (Mn 0.5,Fe 0.5)CO 3. Any point that lies within the triangle represents a solid solution that contains all three end member components. The precise composition of any three‐component solid solution can be determined by the distance from the point to the three apices of the triangle. Point B in Figure 3.3lies closest to the rhodochrosite apex and farthest from the magnesite apex and so clearly contains more Mn than Fe and more Fe than Mg. Its precise composition can be expressed as Sd 10Ms 2Rc 88or as (Fe 0.10,Mg 0.02,Mn 0.88)CO 3. Many other examples of three‐component systems with complete solid solution exist; all may be represented in a similar fashion by their position on a triangular diagram.
3.1.2 Coupled (paired) ionic substitution
Coupled (paired) ionic substitutioninvolves the simultaneous substitution of ions of different charges in two different structural sites in a way that preserves the electrical neutrality of the crystal lattice ( Figure 3.4). The substitution of ions of different charge in one structural site changes the electric charge of the crystal lattice; this requires a second set of substitutions of ions in a second structural site to balance that change in charge. Many examples of coupled ionic substitution exist; none are more important than those that occur in the plagioclase feldspars, the most abundant mineral group in Earth's crust.
In the plagioclase feldspars, similar size ions of sodium (Na +1) and calcium (Ca +2) can substitute for one another in any proportion in the large cation coordination site. However, when calcium (Ca +2) substitutes for sodium (Na +1), the positive charge of the crystal lattice is increased, and when the reverse occurs, the positive charge of the lattice is decreased. These changes in charge are balanced by a second set of substitutions. This second set of substitutions occurs in the small tetrahedral cation coordination site where aluminum (Al +3) and silicon (Si +4) substitute for one another. When a sodium (Na +1) ion is added to the large cation coordination site, a silicon (Si +4) ion is added to the small cation structural site. The two sites together contain a total charge of +5 that is balanced by the anions in the plagioclase structure. When a calcium (Ca +2) is added to the large cation site, an aluminum (Al +3) is added to the small cation site. Once again, the two sites together contain a total charge of +5 which is balanced by the anions in the plagioclase structure. The two substitutions are paired. If a sodium (Na +1) ion replaces a calcium (Ca +2) ion in the first coordination site, a silicon (Si +4) ion must simultaneously replace an aluminum (Al +3) ion in the second structural site for the two sites to total +5, so that the electrical neutrality of the crystal lattice is maintained. Ideally all substitutions are paired and any change in the proportion of sodium to calcium (Na/Ca) in the large ion site is balanced by a similar change in the proportion of silicon to aluminum (Si/Al) in the small ion site. As a result, the general composition of plagioclase can be represented by the formula (Na,Ca)(Si,Al)AlSi 2O 8to emphasize the nature of coupled ionic substitutions.
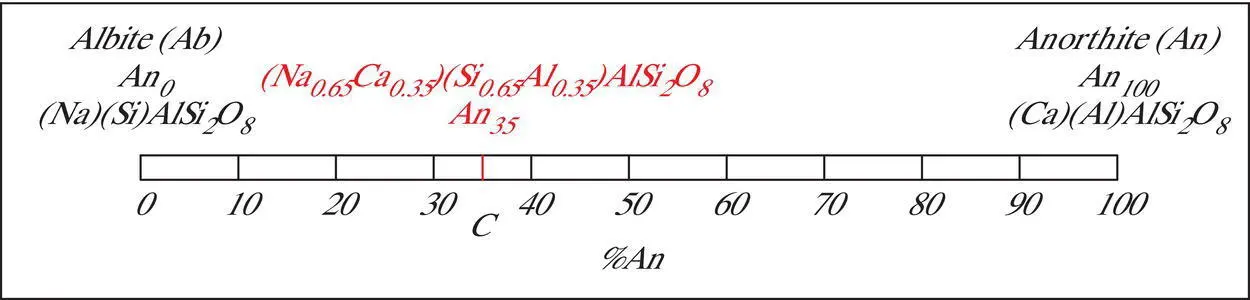
Figure 3.4 Coupled ionic substitution in the plagioclase solid solution series.
The plagioclase group can be represented as a two‐component system with coupled ionic substitution ( Figure 3.4). The two end members are the “pure” sodium plagioclase called albite (Ab ) ,whose formula can be written as (Na)(Si)AlSi 2O 8(or NaAlSi 3O 8), and the “pure” calcium plagioclase called anorthite (An), whose formula can be written as (Ca)(Al)AlSi 2O 8(or CaAl 2Si 2O 8). Since a complete solid solution series exists between these two end members, any plagioclase composition can also be represented by its position on a tie line between the end member components or by the proportions of albite (Ab) and/or anorthite (An). In Figure 3.4, the composition of “pure” sodium plagioclase can be represented by (1) its position on the left end of the tie line, (2) Ab 100, (3) An 0, or (4) the formula [(Na 1.0,Ca 0.0)(Si 1.0,Al 0.0)AlSi 2O 8] = NaAlSi 3O 8. Similarly, the composition of “pure” calcium plagioclase can be represented by (1) its position on the right end of the tie line, (2) Ab 0, (3) An 100, or (4) the formula [(Na 0.0,Ca 1.0)(Si 0.0,Al 1.0)AlSi 2O 8] = CaAl 2Si 2O 8. Plagioclase compositions are generally expressed in terms of anorthite proportions, with the implication that the proportion of albite is (100 − An x). An intermediate plagioclase solid solution such as the composition marked C on the tie line in Figure 3.4has a composition that is represented by its position on the tie line, which can be represented as An 35(=Ab 65). An 35can also be expressed as (Na 0.65,Ca 0.35)(Si 0.65,Al 0.35)AlSi 2O 8. This indicates that 65% of the large cation site is occupied by sodium (Na +1) ions and 35% by calcium (Ca +2) ions with a coupled substitution of 65% silicon (Si +4) and 35% aluminum (Al +3) existing in the small cation site.
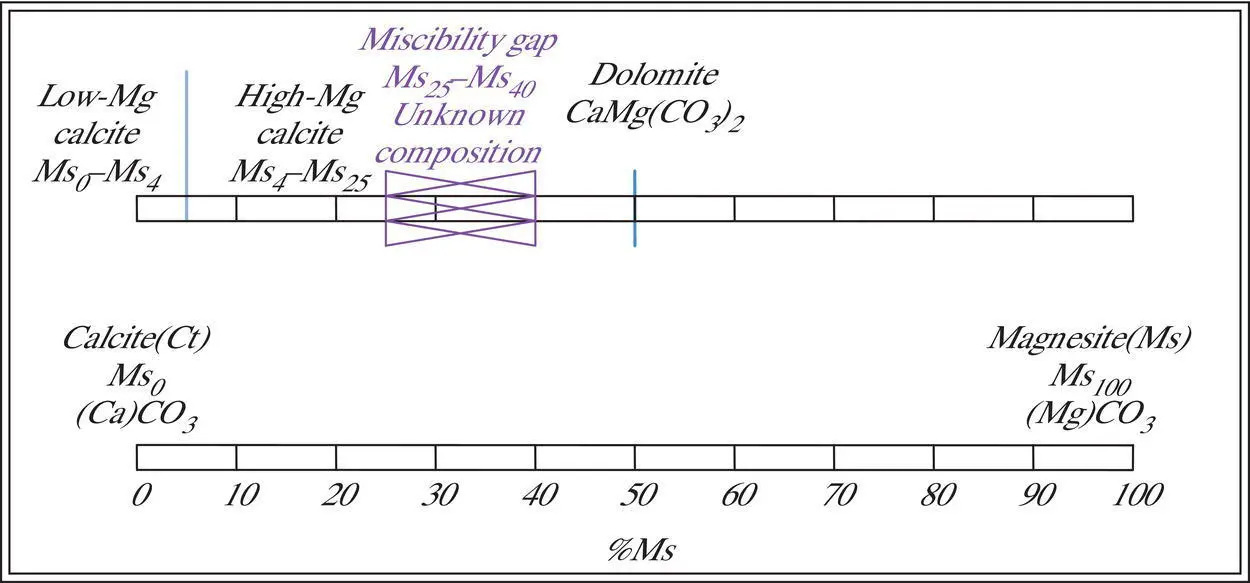
Figure 3.5 Limited substitution and miscibility gap in calcium–magnesium carbonates with compositional ranges of low‐Mg calcite, high‐Mg calcite, and dolomite.
3.1.3 Limited ionic substitution
As noted previously, substitution is limited by significant differences in the ionic radii or charge of substituting ions. Ions of substantially different size limit the amount of substitution so that only a limited solid solutioncan exist between end member components. This situation can be illustrated in the rhombohedral carbonates by the limited solid solution series that exists between calcite (CaCO 3) and magnesite (MgCO 3). Once again, the potential solid solution series can be represented as a line between the two end members, and the composition of any calcium–magnesium‐bearing, rhombohedral carbonate may be represented by a formula, by its position on the tie line or by the proportion of an end member component (calcite = Ct or magnesite = Ms). However, because calcium cations (Ca +2) are more than 30% larger than magnesium (Mg +2) cations, the substitution between the two end members is limited. Because the amount of substitution is limited, many potential compositions do not exist in nature. Such gaps in a solid solution series are called miscibility gapsby analogy with immiscible liquids that do not mix in certain proportions. In this series, a miscibility gap exists between approximately Ms 25= Ct 75and Ms 40= Ct 60( Figure 3.5). To the left of this miscibility gap, a partial solid solution series exists between Ms 0= (Ca 1.0,Mg 0.0)CO 3and Ms 25= (Ca 0.75,Mg 0.25)CO 3. Many organisms secrete shells in this compositional range ( Chapter 14). Within this range, we can define low magnesium calciteand high magnesium calcitein terms of their proportions of calcite (Ct) and magnesite (Ms) end members. Low magnesium calcites generally contain less than 4% magnesium (Mg +2) substituting for calcium (Ca +2) in this structural site and so have compositions in the range Ct 96–100= Ms 0–4( Figure 3.5). High magnesium calcites have more than 4% magnesium substituting for calcium and therefore have compositions in the range Ct 75–96= Ms 4–25. Some workers further subdivide these compositions into medium magnesium and high magnesium calcite with a boundary at 10% magnesium (James and Jones 2016). Compositions from Ms 40–55= Ct 45–60actually have a different structure – that of the double carbonate mineral dolomitewhose average composition is CaMg(CO 3) 2. Many other examples exist of limited substitution series with miscibility gaps. The importance of mineral compositional variations that result from variations in substitution can be more fully understood in the context of phase stability diagrams, as discussed in the following section.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Earth Materials»
Представляем Вашему вниманию похожие книги на «Earth Materials» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Earth Materials» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












