John O'Brien - Earth Materials
Здесь есть возможность читать онлайн «John O'Brien - Earth Materials» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Earth Materials
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Earth Materials: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Earth Materials»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Earth Materials,
Earth Materials,
Earth Materials — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Earth Materials», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
When atoms are ionized by the loss or gain of electrons, their ionic radii, invariably change. This results from the electrical forces that act between the positively charged protons in the nucleus and the negatively charged electrons in the electron clouds. The ionic radii of cations tend to be smaller than the atomic radii of the same element ( Figure 2.7). As electrons are lost from the electron cloud during cation formation, the positively charged protons in the nucleus tend to exert a greater force on each of the remaining electrons. This force draws electrons closer to the nucleus, reducing the effective radius of the electron cloud as it contracts. The larger the charge on the cation, the more its radius is reduced by the excess positive charge in the nucleus. This is well illustrated by the radii of the common cations of iron ( Figure 2.8). Ferric iron (Fe +3) has a smaller radius (0.64 Å) than does ferrous iron (Fe +2= 0.74 Å). Both iron cations possess much smaller radii than neutral iron (Fe 0= 1.23 Å) in which there is no excess positive charge in the nucleus.
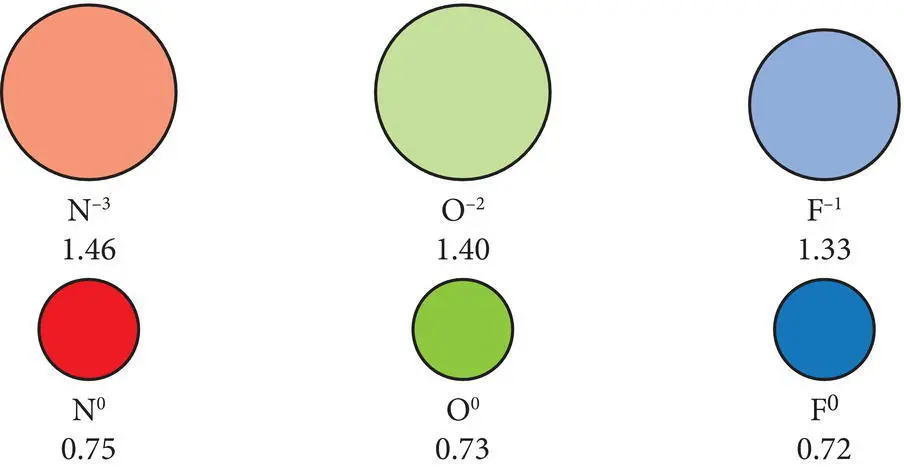
Figure 2.8 Radii (in angstroms) of some common anions in relationship to the atomic radius of the neutral atoms.
The ionic radii of anions are significantly larger than the atomic radii of the same neutral (uncharged) element ( Figure 2.8). When electrons are added to the electron cloud during anion formation, the positively charged protons in the nucleus exert a smaller force on each of the electrons. This allows the electrons to move farther away from the nucleus, which causes the electron cloud to expand, increasing the effective radius of the anion. The larger the charge on the anion, the more its effective radius is increased.
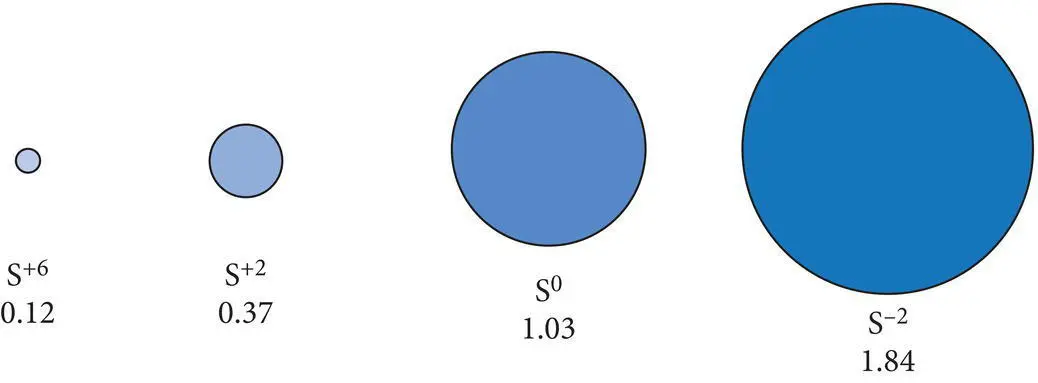
Figure 2.9 Radii (in angstrom units) of some common anions and cations of sulfur in relationship to the neutral atom radius.
The expansion of anions and the contraction of cations are well illustrated by the common ions of sulfur ( Figure 2.9). The divalent sulfur (S −2) anion possesses a relatively large average radius of 1.84 Å. In this case, the two electrons gained during the formation of a divalent sulfur anion produce a large deficit between positive charges in the nucleus and negative charges in the electron cloud. This leads to a significant increase in the effective ionic radius compared to that of electrically neutral sulfur (S 0= 1.03 Å). Neutral sulfur in turn is much larger than the divalent sulfur cation (S +2= 0.37 Å) and the very small, highly charged hexavalent sulfur cation (S +6= 0.12 Å). Keep in mind that the effective radius of a particular anion does vary somewhat. As we will see in the following sections, it depends on the environment in which bonding occurs, the number of nearest neighbors and the type of bond that forms.
2.3 CHEMICAL BONDS
2.3.1 The basics
Atoms in minerals, rocks, and other Earth materials are held together by forces or mechanisms called chemical bonds. The nature of these bonds strongly influences the properties and behavior of these materials. The nature of the bonds is, in turn, strongly influenced by the electron configuration of the elements that combine to produce the mineral, rock or other material.
Five principle bond types and many hybrids occur in minerals. The three most common bond types are (1) ionic, (2) covalent, and (3) metallic. They can be modeled based on the behavior of valence electrons in the outer quantum levels of atoms. During bonding, valence electrons display varying tendencies to change position based on their periodic properties. In discussing chemical bonds, it is useful to divide elements into those that are metallic and those that are nonmetallic.
Ionic bondsinvolve the linking together of metallic and nonmetallic elements, covalent bondsinvolve the linking of two nonmetallic elements, and metallic bondsinvolve the linking of two metallic elements. Hybrids between these bond types are common. Minerals with such hybrid or transitional bonds commonly possess combinations of features characteristic of each bond type. Other bond types include van der Waals and hydrogen bonds. Chemical bonding is a very complicated process; the models used below are simplifications designed to make this complex process easier to understand to a reasonable degree.
Another useful concept for understanding chemical bonds, developed originally by Linus Pauling (1929), is the concept of electronegativity (see Table 2.3). Electronegativity (En)is an empirical measure that expresses the tendency of an element to attract electrons when atoms bond. Highly electronegative elements (En > 3.0) have a strong tendency to attract electrons and become anions during bonding. Many column 16 (group VIA) and column 17 (group VIIA) elements are highly electronegative, requiring capture of two or one electrons, respectively, to achieve a stable electron configuration. Elements with low electronegativity (En < 1.5) are electropositive, metallic elements with a tendency to give up electrons to more electronegative elements during bonding to become positively charged cations. Highly electropositive elements include column 1 (group IA) and column 2 (group IIA) elements that tend to release one or two electrons, respectively, to achieve a stable electron configuration. Electronegativity is a very helpful concept in discussions of how atoms bond to produce larger molecules and minerals.
2.3.2 Ionic (electrostatic) bonds
When very metallic atoms bond with very nonmetallic atoms, an ionic bond,also called an electrostatic bond, is formed. Because the very metallic atoms (e.g., columns 1 and 2) are electropositive, they have a strong tendency to give up one or more electrons to achieve a stable configuration in their highest principal quantum level. In doing so, they become positively charged cations, whose charge is equal to the number of electrons each has lost. At the same time, very nonmetallic atoms (columns 16 and 17) are electronegative and have a strong tendency to gain one or more electrons in order to achieve a stable configuration in their highest principal quantum level. In doing so, they become negatively charged anions, with a charge equal to the number of electrons each has gained. When very metallic and very nonmetallic atoms bond, the metallic atoms give up or donate their valence electrons to the nonmetallic atoms that capture them. It is like a tug‐of‐war in which the electronegative side always wins the battle for electrons. In the electron exchange process, the atoms of both elements develop stable noble element electron configurations while becoming ions of opposite charge. Because particles of opposite charge attract, the cations and anions are held together by the electrostatic attraction between them that results from their opposite charges. Larger clusters of ions form as additional ions exchange electrons and are bonded and crystals begin to grow.

Figure 2.10 (a) Ionic bonding develops between highly electronegative anions and highly electropositive cations. When neutral sodium (Na 0) atoms (red) release an electron to become cations (Na +1) their ionic radius decreases. When neutral chlorine (Cl 0) atoms (blue) capture an electron to become anions (Cl −1) their ionic radius increases. (b) Ions of opposite charge attract to form crystals such as sodium chloride (NaCl).
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Earth Materials»
Представляем Вашему вниманию похожие книги на «Earth Materials» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Earth Materials» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












