John O'Brien - Earth Materials
Здесь есть возможность читать онлайн «John O'Brien - Earth Materials» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Earth Materials
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Earth Materials: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Earth Materials»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Earth Materials,
Earth Materials,
Earth Materials — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Earth Materials», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
The diagonal ruleis a simple rule or memory device that predicts, with very few exceptions, the sequence in which electrons are added to the electron cloud. The order in which electrons are added to shells is depicted by a series of diagonal lines from 1s to 7p ( Figure 2.5).
Table 2.3shows the ground state electron configurations for the elements. One can write the electron configuration of any element in a sequence from lowest to highest energy electrons. For example, calcium (Z = 20) possesses the electron configuration 1s 2, 2s 2, 2p 6, 3s 2, 3p 6, 4s 2. Elements with principal quantum levels (shells) or azimuthal quantum s‐ and p‐subshells that are completely filled (that is they contain the maximum number of electrons possible) possess very stable electron configurations. These elements include the noble gas elements such as helium (He), neon (Ne), argon (Ar), and krypton (Kr) which, because of their stable configurations, tend not to react with or bond to other elements. For elements other than helium, the highest quantum level stable configuration is s2, p 6, sometimes referred to as the “ stable octet. ”
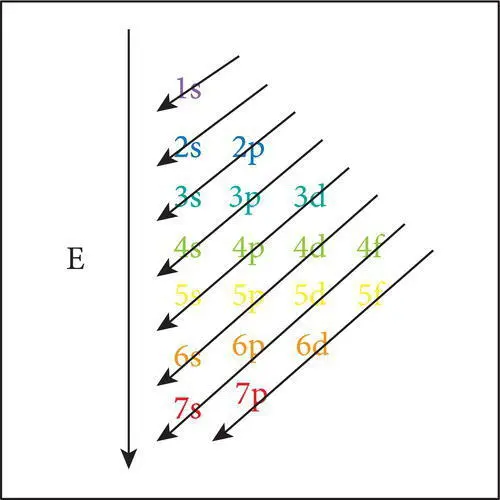
Figure 2.5 The diagonal rule for determining the sequence in which electrons are added to the electron cloud; K‐shell electrons violet; L‐shell blue; M‐shell bluish green; N‐shell green; O‐shell yellow; P‐shell orange and Q‐shell red.
2.2 THE PERIODIC TABLE
The naturally occurring and synthetic elements discovered to date display certain periodic traits; that is, several elements with different atomic numbers display similar chemical behavior. Tables that attempt to portray the periodic behavior of the elements are called periodic tables. It is now well known that the periodic behavior of the elements is related to their electron configurations. In most modern periodic tables ( Table 2.3) the elements are arranged in seven rows or periodsand eighteen columns or groups. Two sets of elements, the lanthanidesand the actinides, which belong to the sixth and seventh rows, respectively, are listed separately at the bottom of such tables to allow all the elements to be shown conveniently on a printed page of standard dimensions. For a rather different approach to organizing the elements in a periodic table for Earth scientists, readers are referred to Railsback (2003).
2.2.1 Rows (periods) on the periodic table
On the left‐hand side of the periodic table the row numbers 1–7 indicate the highest principle quantum level in which electrons occur in the elements in that row. Every element in a given horizontal row has its outermost electrons in the same principle energy region or level. Within each row, the number of electrons increases with the atomic number from left to right. The number of elements in each row varies, and reflects the sequence in which electrons are added to various quantum levels as the atoms are formed. For example, row 1 has only two elements because the first quantum level can contain only two 1s electrons. The two elements are hydrogen (1s 1) and helium (1s 2). Row 2 contains eight elements that reflect the progressive addition of 2s, then 2p electrons during the formation of lithium (helium + 2s 1) through neon (helium + 2s 2, 2p 6). Row 3 contains eight elements that reflect the filling of the 3s and 3p quantum regions respectively during the addition of electrons in sodium (neon + 3s 1) through argon (neon + 3s 2, 3p 6) as indicated in Table 2.3. Row 4 contains 18 elements which reflects the addition of 10 3d‐subshell electrons after the 4s electrons and before the 4p elections. Row 5 contains 32 elements because of the addition of 14 3f electrons after the two 5s electrons prior to the addition 10 4d subshell electrons and the six 5p electrons ( Figure 2.5). The process continues through rows 6 and 7 ending with uranium. The rows that contain up to 14 f‐subshell electrons are too long to fit conveniently in a table, so the elements (lanthanides and actinides) to which f‐subshell electrons have been added are shown separately at the bottom of the table. In summary, elements are grouped into rows on the periodic table according to the highest ground state principle quantum level (1–7) occupied by their electrons. Their position within each row depends on the distribution and numbers of electrons within the principle quantum levels.
Table 2.3 Periodic table of the naturally‐occurring elements displaying atomic symbols, atomic number (Z), average mass, ground state electron configuration, common valence states and electronegativity of each element.
Simplified periodic table of the elements showing symbols atomic numbers and mass numbers 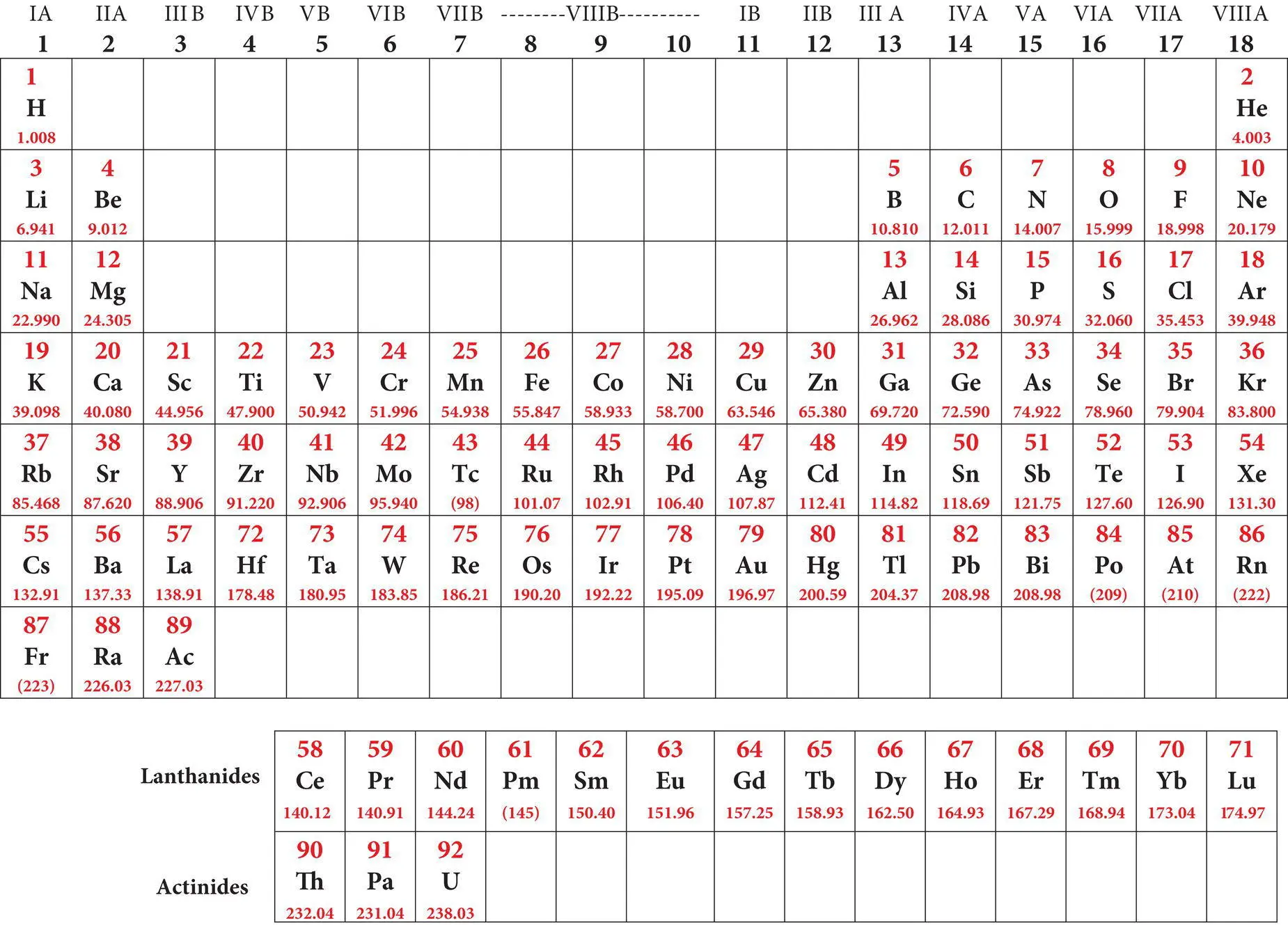 |
Simplified periodic table of the elements showing symbols and electron configurations 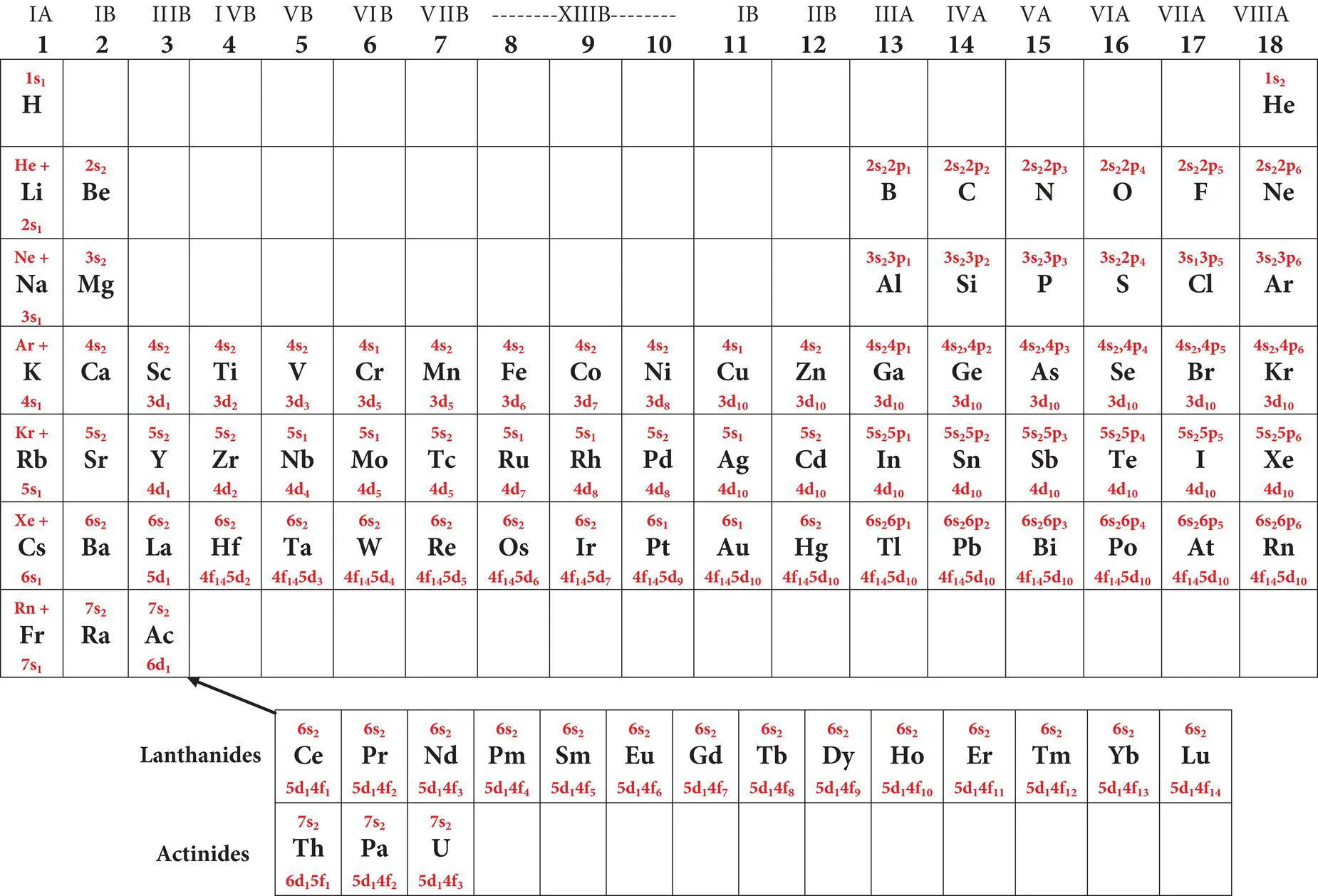 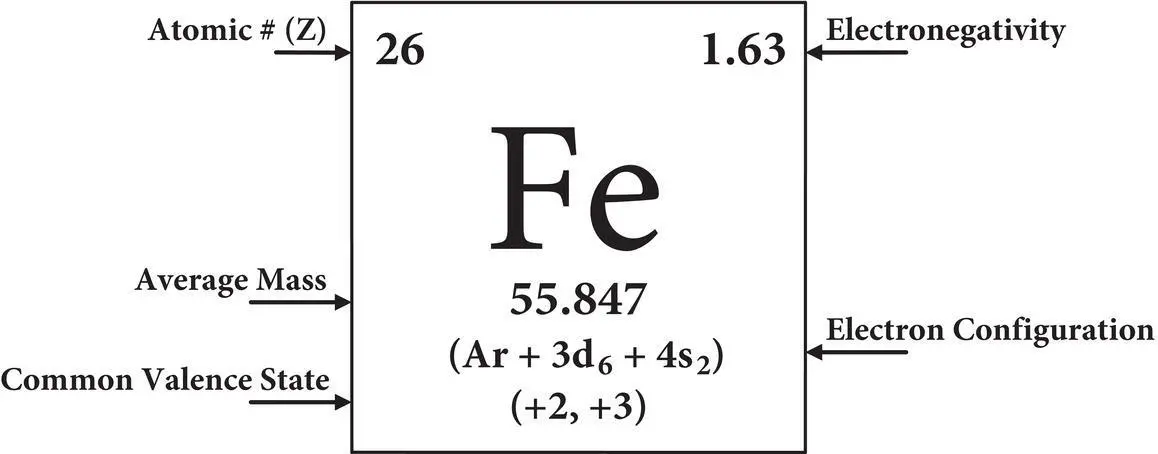 |
Simplified periodic table of the elements electronegativities common and valence states 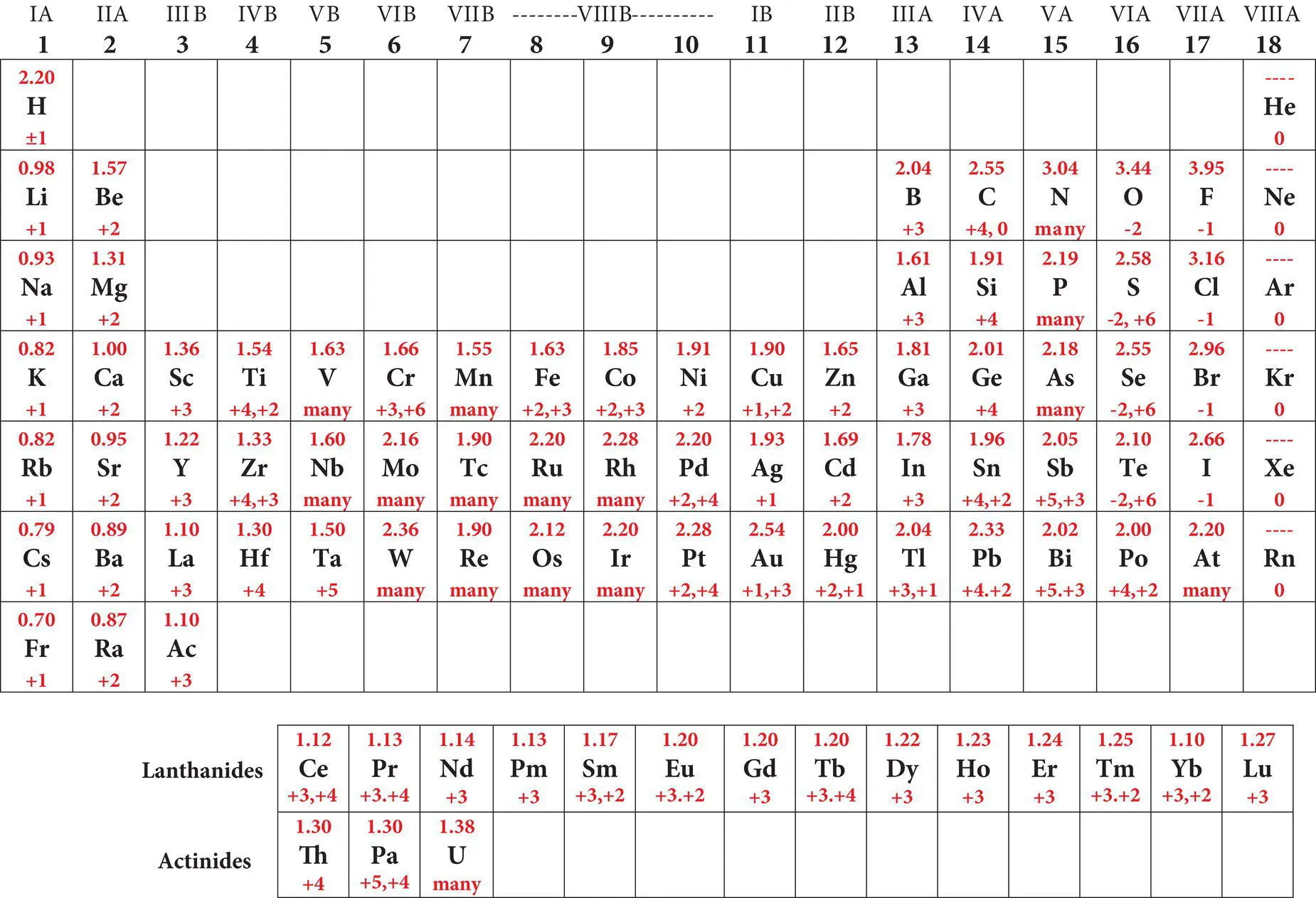 |
2.2.2 Ionization
The periodic table not only organizes the elements into rows based on their electron properties, but also into vertical columns based upon their tendency to gain or lose electrons to become more stable ( Table 2.3). Ideal atoms are electrically neutral because they contain the same numbers of positively charged protons and negatively charged electrons (p += e −). Many atoms are not electrically neutral; instead they are electrically charged particles called ions. The process by which they acquire their charge is called ionization( Box 2.1). In order for an ion to form, the number of positively charged protons and negatively charged electrons must become unequal. Cationsare positively charged ions because they have more positively charged protons than negatively charged electrons (p +> e −). Their charge is equal to the number of excess protons (p +− e −). Cations form when electrons are lost from the electron cloud. Ions that have more negatively charged electrons than positively charged protons, such as the ion chlorine (Cl −), will have a negative charge and are called anions. The charge of an anion is equal to the number of excess electrons (e −− p +). Anions form when electrons are added to the electron cloud during ionization.
Box 2.1Ionization energy
Ionization energy (IE)is the amount of energy required to remove an electron from its electron cloud. Ionization energies are periodic as illustrated for 20 elements in Table B2.1.
The first ionization energy is the amount of energy required to remove one electron from the electron cloud; the second ionization energy is the amount required to remove a second electron and so forth. Ionization energies are lowest for electrons that are weakly held by the nucleus and higher for electrons that are strongly held by the nucleus or are in stable configurations. Ionization energies decrease down the periodic table because the most weakly held outer electrons are shielded from the positively charged nucleus by a progressively larger number of intervening electrons. Elements with relatively low first ionization energies are called electropositive elementsbecause they tend to lose one or more electrons and become positively charged cations. Most elements with high first ionization energies are electronegative elementsbecause they tend to add electrons to their electron clouds and become negatively charged anions. Since opposite charges tend to attract, you can imagine the potential such ions have for combining to produce other Earth materials. The arrangement of elements into vertical columns or groups within the periodic table helps us to comprehend the tendency of specific atoms to lose, gain or share electrons. For example, on the periodic table (see Table 2.3), column 2 (IIA) elements commonly exist as divalent (+2) cations because the first and second ionization energies are fairly similar and much lower than the third and higher ionization energies. This permits two electrons to be removed fairly easily from the electron cloud, but makes the removal of additional electrons much more difficult. Column 13 (IIIA) elements commonly exist as trivalent (+3) cations (see Table 2.3). These elements have somewhat similar first, second and third ionization energies, which are much smaller than the fourth and higher ionization energies. The transfer of electrons is fundamentally important in the understanding of chemical bonds and the development of mineral crystals.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Earth Materials»
Представляем Вашему вниманию похожие книги на «Earth Materials» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Earth Materials» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












