John O'Brien - Earth Materials
Здесь есть возможность читать онлайн «John O'Brien - Earth Materials» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Earth Materials
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Earth Materials: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Earth Materials»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Earth Materials,
Earth Materials,
Earth Materials — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Earth Materials», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
30 Sheridan, R. (1987). Pulsation tectonics as the control of long‐term stratigraphic cycles. Paleoceanography 2: 97–118.
31 Stern, R.A. and Bleeker, W. (1998). Age of the world's oldest rocks refined using Canada's SHRIMP: the Acasta Gneiss Complex, Northwest Territories, Canada. Geoscience Canada 25: 27–31.
32 Tarduno, J., Bunge, H.P., Sleep, N., and Hansen, U. (2009). The bent Hawaiian–Emperor hotspot track: inheriting the mantle wind. Science 324: 50–53.
33 Torsvik, T., Doubrovine, P.V., Steinberger, B. et al. (2017). Pacific plate motion change caused the Hawaiian‐Emperor Bend. Nature Communications 8 https://doi.org/10.1038/ncomms15660.
34 Tschauner, O., Ma, C., Beckett, J.R. et al. (2014). Discovery of bridgmanite, the most abundant mineral in Earth, in a shocked meteorite. Science 346: 1100–1102.
35 Valley, J.W., Cavosie, A.J., Ushikubo, T. et al. (2014). Hadean age for a post‐magma‐ocean zircon confirmed by atom probe tomography. Nature Geoscience 7: 219–223.
36 Vine, F.J. and Matthews, D.H. (1963). Magnetic anomalies over ocean ridges. Nature 199: 947–949.
37 Wang, T., Song, X., and Han, H.X. (2015). Equatorial anisotropy in the inner part of Earth's core from autocorrelation of earthquake coda. Naure Geoscience 3: 224–227.
38 Wang, C., Gordon, R.C., and Zhang, T. (2017). Bounds of geologically current rates of motion of groups of hot spots. Geophysical Research Letters 44: 6048–6056.
39 Wilde, S., Valley, J.W., Peck, W.H., and Graham, C.M. (2001). Evidence from detrital zircons for the existence of continental crust and ocean on the Earth 4.4Ga ago. Nature 409: 175–178.
40 Williams, Q. and Garnero, E.J. (1996). Seismic evidence for partial melt at the base of Earth's mantle. Science 273: 1528–1530.
41 Wilson, J.T. (1963). Evidence from islands on the spreading of ocean floors. Nature 197: 536–538.
42 Wilson, J.T. (1965). A new class of faults and their bearing on continental drift. Nature 207: 343–347.
Chapter 2 Atoms, elements, bonds, and coordination polyhedra
1 2.1 Atoms
2 2.2 The periodic table
3 2.3 Chemical bonds
4 2.4 Pauling's rules and coordination polyhedra
5 2.5 The chemical classification of minerals
If we zoom in on any portion of Earth, we will see that it is composed of progressively smaller entities. At very high magnification, we will be able to discern very small particles called atoms. Almost all Earth materials are composed of atoms which, in turn, strongly influence their material properties. Understanding the ways in which these basic chemical constituents combine to produce larger scale Earth materials is essential to understanding our planet.
In this chapter we will consider the fundamental chemical constituents that bond together to produce Earth materials such as minerals and rocks. We will discuss the nuclear and electron configurations of atoms and then discuss the role these play in determining both atomic and mineral properties and the conditions under which minerals form. This information will provide a basis for understanding how and why minerals, rocks and other Earth materials have the following characteristics:
1 They possess specific properties that characterize and distinguish them.
2 They provide benefits and hazards through their production, refinement and use.
3 They form in response to particular sets of environmental conditions and processes.
4 They record information about the conditions and processes that produce them.
5 They permit us to infer significant events in Earth's history.
2.1 ATOMS
Earth materials are composed of smaller entities. At very small scales, such as viewed through a tunneling electron microscope, we are able to discern particles called atomswhose effective diameters are a few angstroms (1 Å = 10 −10m, about a million times smaller than the width of a human hair). These tiny atoms, in turn, consist of three main particles – electrons, protons, and neutrons – which were discovered between 1895 and 1902. The major properties of electrons, protons, and neutrons are summarized in Table 2.1.
Protons (p + )and neutrons (n0)each have a mass of ~1 atomic mass unit (amu) and are clustered together in a small, positively charged, central region of the atom called the nucleus( Figure 2.1). Protons possess a positive electric charge and neutrons are electrically neutral. The nucleus is surrounded by a vastly larger, mostly “empty”, region called the electron cloud. The electron cloud represents the area in which the electrons (e − )in the atom move about the nucleus in patterns called orbitals ( Figure 2.1). Electrons have a negative electric charge and an almost negligible mass of 0.000 005 amu. Knowledge of these three fundamental particles in atoms is essential to understanding how minerals and other materials form, how they can be used as resources and how we can deal with their hazardous effects that can be seen occasionally.
Table 2.1 Major properties of electrons, protons, and neutrons.
| Particle type | Electric charge | Atomic mass (amu) a |
|---|---|---|
| Proton (p +) | +1 | 1.00 728 |
| Neutron (n 0) | 0 | 1.00 867 |
| Electron (e −) | −1 | 0.0 000 054 |
aamu = atomic mass unit = 1/12 mass of an average carbon atom.
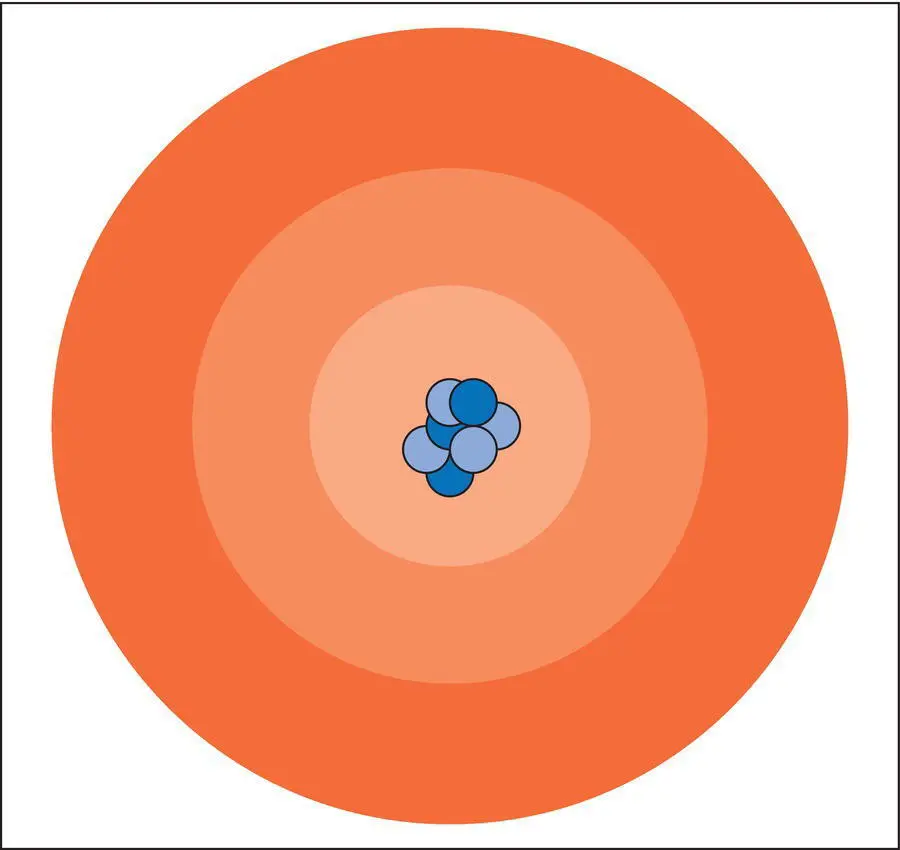
Figure 2.1 Simplified model atom with nucleus that contains positively charged protons (dark blue) and electrically neutral neutrons (light blue) surrounded by an electron cloud (red shades) in which negatively charged electrons move in orbitals about the nucleus.
2.1.1 The nucleus, atomic number, atomic mass number, and isotopes
The nucleus of atoms is composed of positively charged protons and uncharged neutrons bound together by a strong force. Ninety‐two fundamentally different kinds of atoms called elements have been discovered in the natural world. More than 25 additional elements have been created synthetically in laboratory experiments during the past century. Each elementis characterized by the number of protons in its nucleus. The number of protons in the nucleus is called the atomic number (Z). The atomic number is typically represented by a subscript number to the lower left of the element symbol. The 92 naturally occurring elements range from hydrogen (Z = 1) through uranium (Z = 92). Hydrogen ( 1H) is characterized by having one proton in its nucleus. Every atom of uranium ( 92U) contains 92 protons in its nucleus. The atomic number is the unique property that distinguishes the atoms of each element from atoms of all other elements.
Every atom also possesses mass that largely results from the protons and neutrons in its nucleus. The mass of a particular atom is called its atomic mass number, and is expressed in atomic mass units (amu). As the mass of both protons and neutrons is ~1 amu, the atomic mass number is closely related to the total number of protons plus neutrons in its nucleus. The simple formula for atomic mass number is: atomic mass number = number of protons plus number of neutrons (#p ++ #n 0). The atomic mass number is indicated by a superscript number to the upper left of the element symbol. For example, most oxygen atoms have eight protons and eight neutrons so their atomic mass number is written 16O.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Earth Materials»
Представляем Вашему вниманию похожие книги на «Earth Materials» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Earth Materials» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












