John O'Brien - Earth Materials
Здесь есть возможность читать онлайн «John O'Brien - Earth Materials» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Earth Materials
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Earth Materials: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Earth Materials»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Earth Materials,
Earth Materials,
Earth Materials — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Earth Materials», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Although each element has a unique atomic number, many elements are characterized by atoms with different atomic mass numbers. Atoms of the same element that possess different atomic mass numbers are called isotopes. For example, three different isotopes of hydrogen exist ( Figure 2.2a). All hydrogen ( 1H) atoms have an atomic number of 1. The common form of hydrogen atom, sometimes called protium, has one proton and no neutrons in the nucleus; therefore protium has an atomic mass number of 1, symbolized as 1H. A less common form of hydrogen called deuterium, used in some nuclear reactors, has an atomic mass number of 2, symbolized by 2H. This implies that it contains one proton and one neutron in its nucleus (1p ++ 1n 0). A rarer isotope of hydrogen called tritiumhas an atomic mass number of 3, symbolized by 3H. The nucleus of tritium has one proton and two neutrons. Similarly oxygen occurs in three different isotopes: 16O, 17O, and 18O. All oxygen atoms contain eight protons but neutron numbers vary between 16O, 17O, and 18O, which contain eight, nine, and ten neutrons, respectively ( Figure 2.2a). The average atomic mass for each element is the weighted average for all the isotopes of that element. This helps to explain why the listed atomic masses for each element do not always approximate the whole numbers produced when one adds the number of protons and neutrons in the nucleus of a particular isotope.
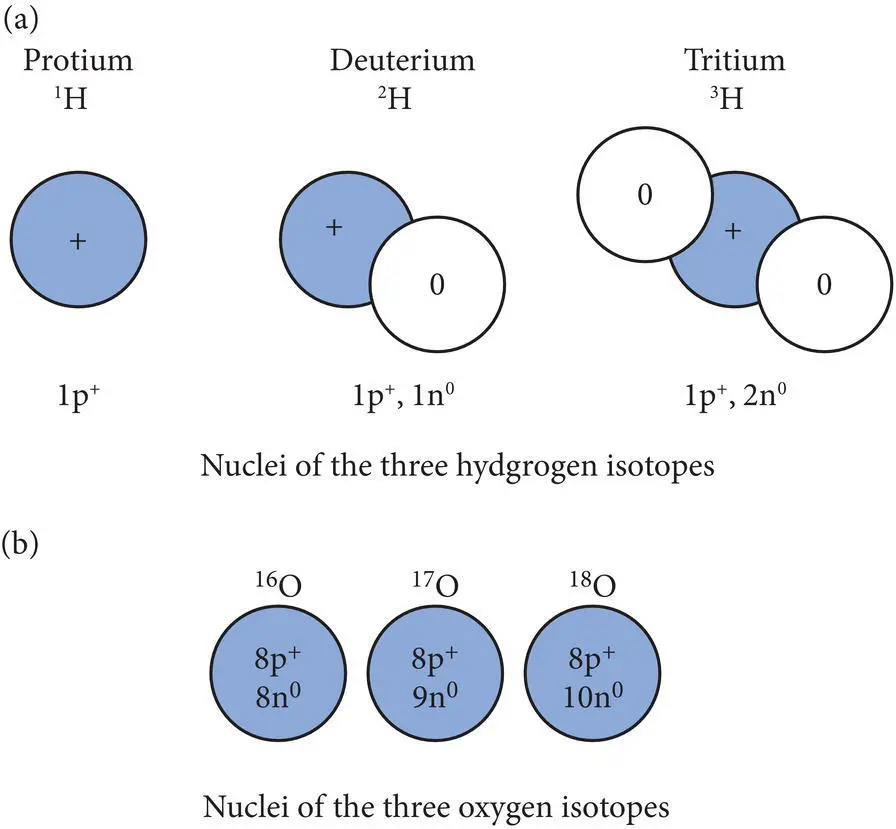
Figure 2.2 (a) Nuclear configurations of the three common isotopes of hydrogen. (b) Nuclear configurations of the three common isotopes of oxygen.
The general isotope symbol for the nucleus of an atom expresses its atomic number to the lower left of its symbol, the number of neutrons to the lower right and the atomic mass number (number of protons + number of neutrons) to the upper left. For example, the most common isotope of uranium has the symbolic nuclear configuration of 92 protons + 146 neutrons and an atomic mass number of 238:
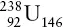
Stable isotopeshave stable nuclear configurations that tend to remain unchanged; they retain the same number of protons and neutrons over time. On the other hand, radioactive isotopeshave unstable nuclear configurations (numbers of protons and neutrons) that spontaneously change over time via radioactive decay processes, until they achieve stable nuclear configurations and become stable isotopes of another element. Both types of isotopes are extremely useful in solving geological and environmental problems, as discussed in Chapter 3. Radioactive isotopes are used in many medical treatments, but also present serious environmental hazards ( Chapter 19).
2.1.2 The electron cloud
Electrons are enigmatic entities, with properties of both particles and wave energy, that move very rapidly around the nucleus in ultimately unpredictable paths. Our depiction of the electron cloud is based on the probabilities of finding a particular electron at a particular place. The wave‐like properties of electrons help to define the three‐dimensional shapes of their probable locations, known as orbitals. The size and shape of the electron cloud defines the chemical behavior of atoms and ultimately the composition of all the Earth materials they combine to form. Simplified models of the electron cloud depict electrons distributed in spherical orbits around the nucleus ( Figure 2.3); the reality is much more complex. Because the electron cloud largely determines the chemical behavior of atoms and how they combine to produce Earth materials, it is essential to understand some fundamental concepts about it.
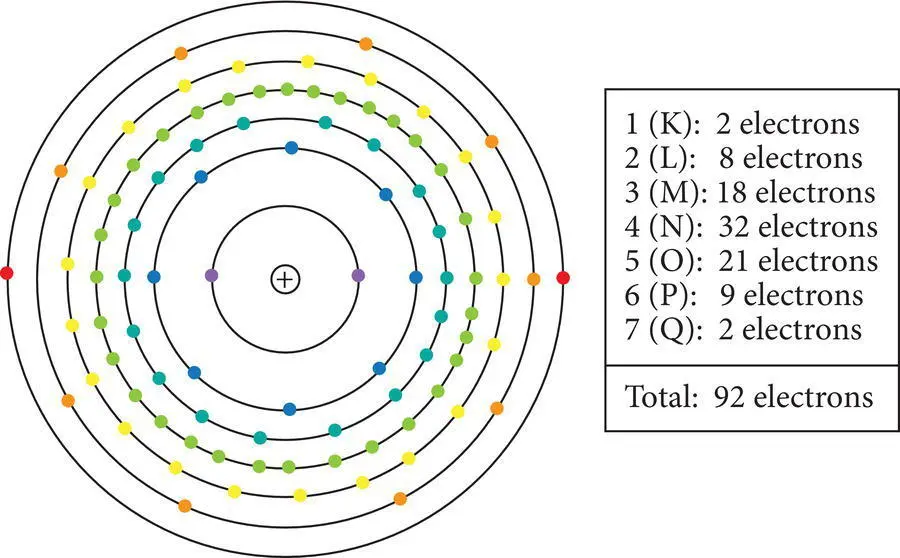
Figure 2.3 Distribution of electrons in the principal quantum levels (“electron shells”) of uranium: K‐shell electrons violet; L‐shell blue; M‐shell bluish green; N‐shell green; O‐shell yellow; P‐shell orange and Q‐shell red.
Every electron in an atom possesses a unique set of properties that distinguishes it from all the other electrons in that atom. An individual electron's identity is given by four properties that include its (1) principal quantum number, (2) azimuthal quantum number, (3) magnetic quantum number, and (4) spin number. Each electron in the electron cloud possesses a unique combination of the four quantum properties.
The principal quantum number (n)signifies the principal quantum energy region,sometimes called “ level” or “ shell ” in which a particular electron occurs. It is related to its distance from the nucleus. Principle quantum regions are numbered in order of increasing electron energies 1, 2, 3, 4, 5, 6 or 7or alternatively lettered K, L, M, N, O, P or Q. These are arranged from low principal quantum number for low energy regions closer to the nucleus to progressively higher quantum number for higher energy regions farther away from the nucleus.
Each principal quantum region or level contains electrons with one or more azimuthal or angular momentum quantum numberswhich signify the directional quantum energy region, sometimes called “ subshell ”, in which the electron occurs. This is related to the angular momentum of the electron and the shape of its orbital. Azimuthal quantum numbers or subshells are labeled s, p, d, and f. As discussed below, the number of electrons in the s and p “subshells” of the atom's highest principal quantum level largely determines the chemical behavior of elements. All four azimuthal quantum numbers are important for understanding the layout of the periodic table of the elements which is discussed below. The other two quantum properties, the magnetic quantum number (m)and the spin number (±½)define the orientation of the quantum probability region in which the electron is located and its spin relative to a reference framework. Table 2.2summarizes the quantum properties of the electrons that can exist in principle quantum regions or “shells” 1–7. To summarize, each electron in an atom possesses a unique set of the four principle quantum properties.
Atomic nuclei were created largely during the “big bang,” by subsequent fusion reactions between protons and neutrons in the interior of stars, and in supernova. When elements are formed, electrons are added to the lowest available quantum level in numbers equal to the number of protons in the nucleus. Electrons are added to the atoms in a distinct sequence, from lowest quantum level electrons to highest quantum level electrons. The relative quantum energy of each electron is shown in Figure 2.4.
Table 2.2 Quantum designations of electrons in the 92 naturally occurring elements. The numbers refer to the principal quantum region occupied by the electrons within the electron cloud; small case letters refer to the subshell occupied by the electrons.
| Principal quantum number | Subshell description | Number of electrons |
|---|---|---|
| 1 (K) | 1s | 2 |
| 2 (L) | 2s | 2 |
| 2p | 6 | |
| 3 (M) | 3s | 2 |
| 3p | 6 | |
| 3d | 10 | |
| 4 (N) | 4s | 2 |
| 4p | 6 | |
| 4d | 10 | |
| 4f | 14 | |
| 5 (O) | 5s | 2 |
| 5p | 6 | |
| 5d | 10 | |
| 5f | 14 | |
| 6 (P) | 6s | 2 |
| 6p | 6 | |
| 7 (Q) | 7s | 2 |
| Total = 92 |

Figure 2.4 The quantum properties of electrons in the 92 naturally occurring elements, listed with increasing quantum energy (E) from bottom to top; K‐shell electrons violet; L‐shell blue; M‐shell bluish green; N‐shell green; O‐shell yellow; P‐shell orange and Q‐shell red.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Earth Materials»
Представляем Вашему вниманию похожие книги на «Earth Materials» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Earth Materials» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












