Source: Panels (b) and (c) are adapted with permission from Ref. [66] (Copyright 2016 John Wiley and Sons).
(D) Chemical structure of 43and 44. (E) CLSM images of A549 cells incubated with 43and 44, respectively.
Source: Reprinted from Ref. [67] (Copyright 2016 American Chemical Society).
(F) Chemical structure of 45. (G) Bright‐field and fluorescent images of E‐coli incubated with 45.
Source: Reprinted from Ref. [65] (Copyright 2016 John Wiley and Sons).
A few numbers of SSB probes were also applied in bacterial imaging. For example, 45is an amphiphilic SSB designed for light‐up detection of anionic surfactants. Due to the positive polarity of the quaternary ammonium salt, the probe can also be used for wash‐free imaging of bacteria enveloped by a negatively charged outer membrane. As Figure 3.24G shows, 45performs high affinity to Escherichia coli and imaging with excellent contrast ratio.
Liu's group [63] also reported another SSB probe with “AIE + ESIPT” characteristic, which is based on a zinc‐coordinated salicylaldehyde hydrazine backbone for apoptotic cell membranes, LDs, and bacterial imaging. As Figure 3.25b shows, lipophilic 46displays favorable affinity to LDs in both live and apoptotic HeLa cells. However, after complexation reaction with zinc perchlorate hexahydrate ( Figure 3.25a), 47becomes strongly hydrophilic even with exceptional water solubility, thus exhibiting a weak emission in water and high emission in water–THF mixtures with THF fractions higher than 80%. 46and 47displayed large Stokes shifts of 215 and 190 nm, respectively, with no overlap between the absorption and emission spectra. As illustrated in Figure 3.25a, since early apoptosis is characterized by partially exposed phosphatidylserine (PS) on the cell surface, positively charged probes can bind to PS to enhance fluorescence emission. For late‐stage apoptotic cells, the membrane integrity is completely compromised; 47was found to light up nuclei specifically. As Figure 3.25c shows, 47only stained the cytoplasm membrane of cells in early‐stage apoptosis induced by 1 μM staurosporine, while no obvious fluorescence signal was observed for healthy cells. The simple manipulation of the presence of metal ions can bring great changes to the properties of the probe and therefore lead to the alteration of the probe function for different applications. Another research also studied the photosensitization property of 47and found its attractive performance in selective imaging and photodynamic extirpating of both Gram‐positive and Gram‐negative bacteria over mammalian cells [64]. As Figure 3.25d and e displays, due to the electrostatic interaction between the positively charged probe and the bacterial membrane, which has a more negative potential, 47only emitted a strong green fluorescence upon incubating with bacteria (gram‐positive Bacillus subtilis or gram‐negative E. coli ), yet showed nearly no signal with mammalian cells Jurkat T or K562. The probe was found to kill Gram‐positive bacteria due to depolarization of the bacterial membrane even in the dark. For Gram‐negative bacteria, 47could generate ROS after white light irradiation for selective photodynamic killing.
3.3 Fluorescent Materials
3.3.1 Solid Fluorescence Emitting and Stimuli‐Responsive Materials
Organic solid fluorescent materials apply widely in organic light‐emitting diodes (OLEDs), photovoltaic devices, organic semiconductor lasers, fluorescent sensors, data storage, security printing, and anticounterfeit materials. Most conventional fluorescent molecules undergo fluorescence quenching in their aggregated state, and improvement of emission quantum yield and brightness are limited when designing for solid fluorescent materials. In contrast, the fluorescence enhancement of AIE molecules in the aggregated state has promoted their development in the field of solid fluorescent materials.
SSB molecules exhibit the characteristics of ESIPT. On the one hand, their large Stokes shift weakens the self‐quenching effect and results in high quantum yields [68]. On the other hand, the ESIPT process can occur rapidly even at low temperature [69], which shows powerful advantages of these SSB molecules as solid fluorescent materials. Furthermore, SSB molecules usually show dual‐color emission and the fluorescence is susceptible to the foreign stimuli factors such as light, heat, mechanical forces, and organic vapor fumigation due to the variation of stacking mode and molecular arrangement in the solid sates, so it has great potential as stimulus‐responsive fluorescence sensing materials.
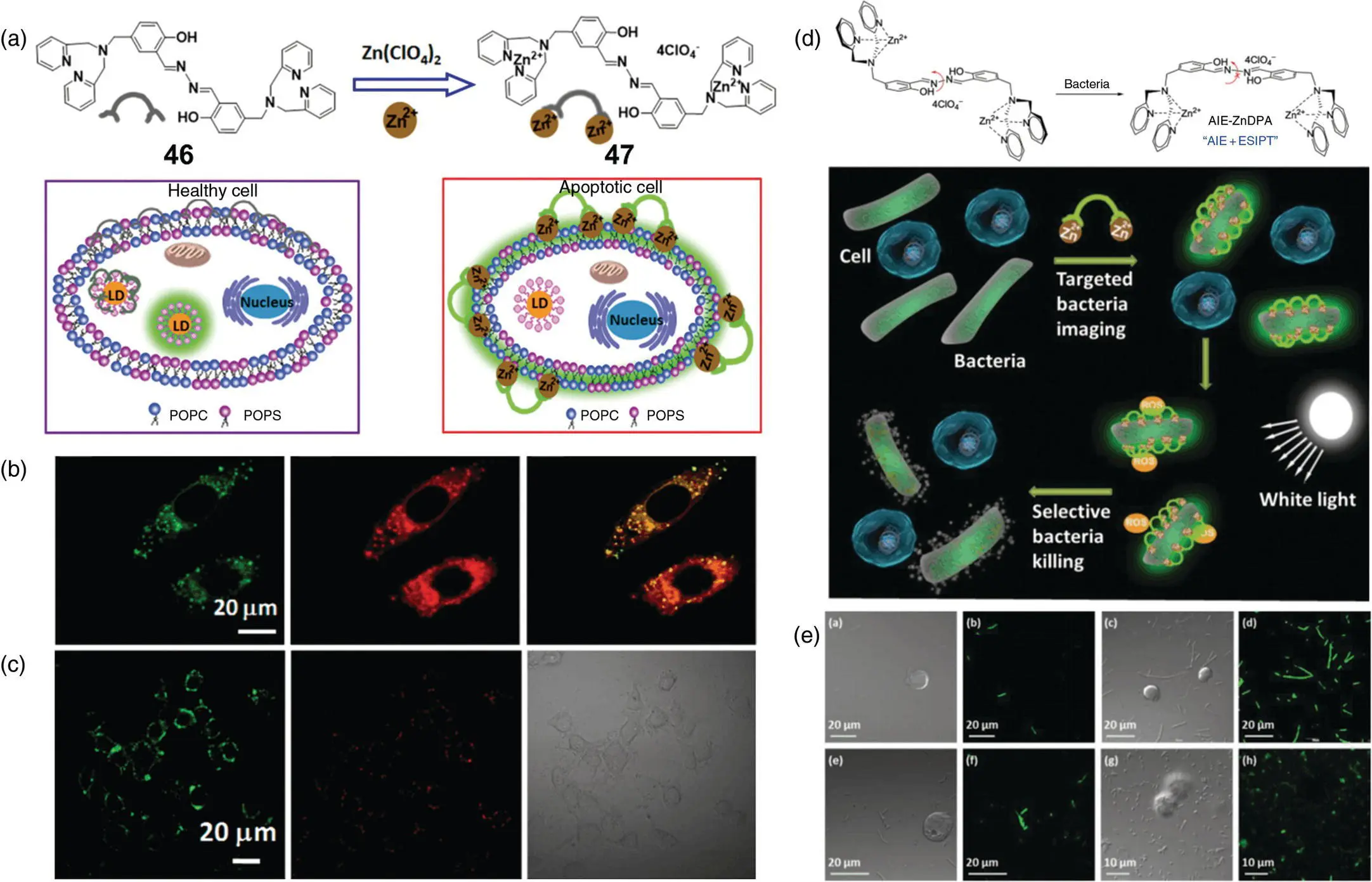
Figure 3.25 (a) Chemical structures of 46and 47, and a schematic illustration of 46for intracellular LD staining in healthy cell and 47for the detection and membrane staining in apoptotic cells. (b) Confocal images of HeLa cells stained with 20 μM 46and costaining with 0.5 μM Nile Red. cConfocal images of early‐stage apoptotic HeLa cells induced by 1 μM staurosporine for two hours, followed by incubation with 20 μM 47for 30 minutes at 37 °C and stained with propidium iodide.
Source: Panels (a–c) are adapted with permission from Ref. [63] (Copyright 2015 American Chemical Society).
(d) Schematic illustration of 47for selective targeting, imaging, and killing of bacteria over mammalian cells. (e) CLSM images of cells and bacteria incubated with 20 μM 47.
Source: Adapted with permission from Ref. [64] (Copyright 2015 John Wiley and Sons).
A series of p ‐carboxyl‐ N ‐salicylideneaniline derivatives with different solid morphologies and emission colors were reported in 2011 by Tong's group [70] ( Figure 3.26a). With different substituents of salicylaldehyde, the fluorescence of these derivatives in their crystalline states shows green to orange emission ( λ em= 518 nm/556 nm). X‐ray crystal structure analysis reveals one‐dimensional microrods obtained with carboxyl substitution on the para‐position of aniline due to the promoted formation of intermolecular hydrogen bonds compared with chlorine substitution ( Figure 3.26b–e). The microrods also show good optical waveguide property owing to the orderly arrangement and transparency. No matter where the location site of excitation is, the transmission of the excited fluorescence to both ends in a one‐dimensional direction was observed ( Figure 3.26f).
Yang's group reported a class of molecules 54with AIE and ESIPT properties in 2012 [71]. The fluorescence intensity of 54in water or powder is significantly enhanced compared to THF solution ( Figure 3.26g). X‐ray crystal structure analysis showed that 54 exhibited a J‐type aggregate, and the N⋯π interaction of the N atom in the thiophene ring with the adjacent thiophene ring of another molecule stabilizes this aggregation form ( Figure 3.26h). In addition, the cis – trans tautomerism of the keto structure is hindered when the molecules are closely packed. Based on these properties, 54was used as a light‐emitting layer to form a simple three‐layer nondoped OLED device with higher color purity and lower efficiency roll‐off.
Читать дальше













